
Cannizzaro reaction is not shown by:
(A)
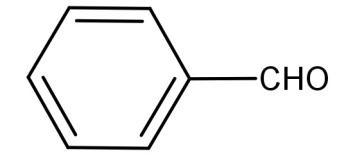
(B)
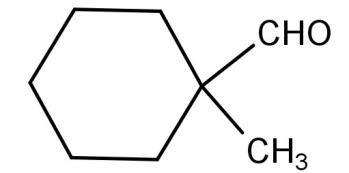
(C) $C{H_3}CHO$
(D) $HCHO$
Answer
232.8k+ views
Hint: Cannizzaro reaction is undergone by aldehydes. It converts aldehydes into a carboxylic acid molecule and a primary alcohol. For this reaction to occur the aldehyde should not contain any alpha hydrogen atoms.
Complete step by step solution:
-First of all, we will see what the Cannizzaro reaction is all about.
The Cannizaro reaction is named after Stanislao Cannizzaro when he succeeded in obtaining benzyl alcohol and potassium benzoate from Benzaldehyde in 1853. This reaction involves the simultaneous oxidation and reduction of aldehyde to yield a carboxylic acid and a primary alcohol. Or we can say that Cannizzaro Reaction explains the method to get one molecule of alcohol and one molecule of carboxylic acid from two molecules of a given aldehyde. This reaction involves nucleophilic acyl substitution on an aldehyde molecule and the leaving group will attack another aldehyde.
-We will now talk about how this reaction proceeds or its mechanism. It basically occurs in 3 steps:
Step-1: In this step, the carbonyl group of the aldehyde is attacked by a nucleophile (like the hydroxide ion ($O{H^ - }$)) causing a disproportionation reaction which leads to the formation of an anion which has two negative charges.
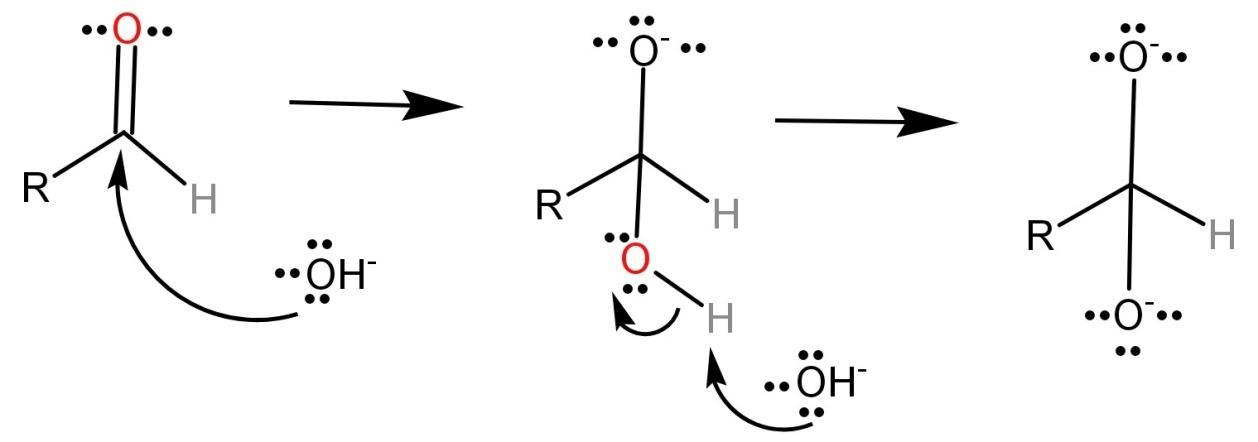
Step-2: The dianion formed in the first step will now function as a hydride reducing agent. The dianion is unstable by nature and thus releases a hydride anion which reacts with another aldehyde. This converts the dianion into a carboxylate anion and the aldehyde into an alkoxide anion.
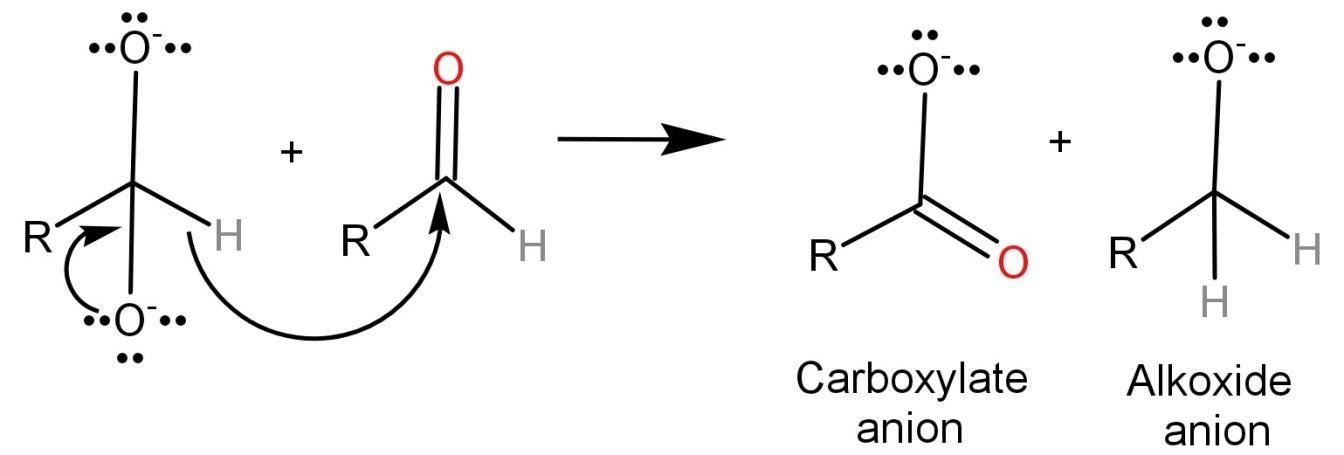
Step-3: In this step, the alkoxide anion gains a proton from water and leads to the formation of alcohol because the alkoxide anion is more basic than water. The carboxylate anion reacts with acid workup to form a carboxylic acid. Here we use an acid workup because carboxylate anion is less basic than water and thus it cannot take a proton from water.
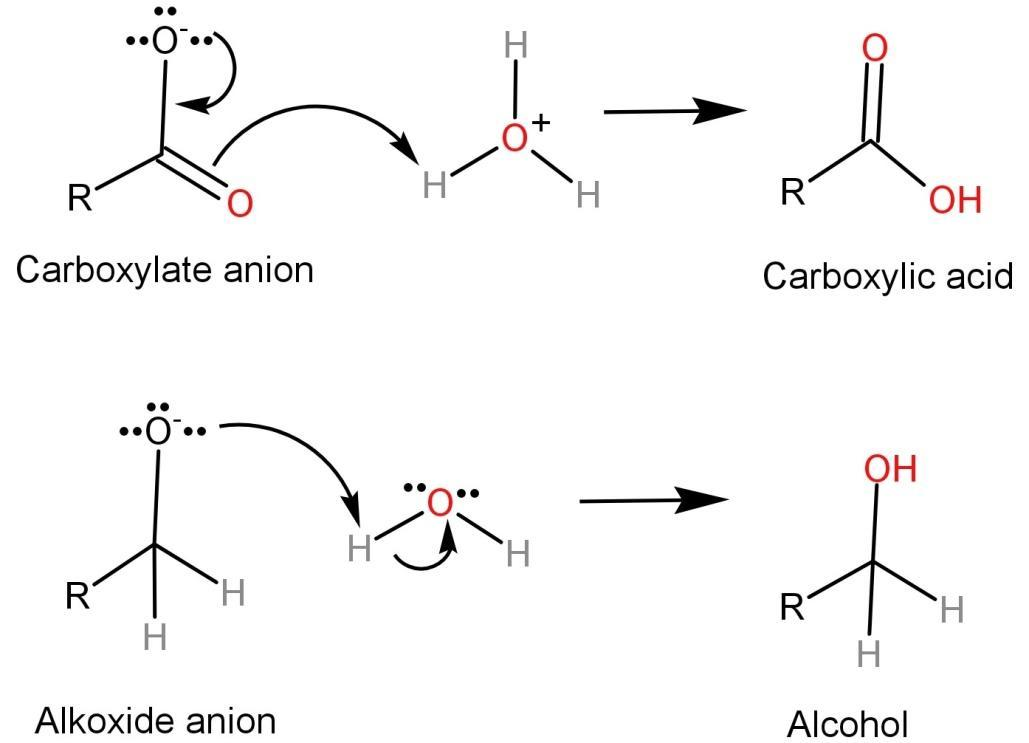
-One of the most important things we should keep in mind about Cannizzaro reaction is that only those compounds undergo Cannizzaro reaction which does not have any alpha hydrogen atoms. Only such compounds will undergo oxidation and reduction or disproportion reaction to yield carboxylic acid and alcohol.
We should know that alpha hydrogen is the hydrogen atom attached to the alpha carbon. And the alpha carbon is the carbon atom present adjacent to the functional group carbon atom.
-We will now observe each option and check who all are capable of undergoing Cannizzaro reaction. We will check them regarding the presence or absence of alpha hydrogens and that aldehyde which does not contain alpha hydrogen will only give Cannizzaro reaction.
For (A):
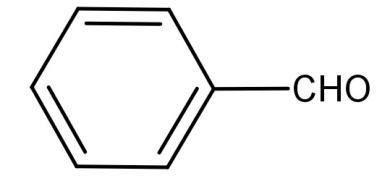
The above compound is benzaldehyde (${C_6}{H_5}CHO$) and it has no alpha hydrogen atoms, so it will undergo Cannizzaro reaction.
For (B):
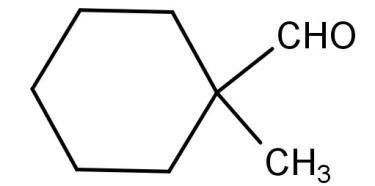
The above structure also does not have any alpha hydrogen atoms attached. So, it will also undergo a Cannizzaro reaction.
For (C) $C{H_3}CHO$: The above compound acetaldehyde has 3 alpha hydrogen atoms attached to the alpha carbon. So, from this, we can say that this compound will not undergo a Cannizzaro reaction.
For (D) HCHO: This compound is known as formaldehyde. It also doesn’t have alpha carbon and so no alpha hydrogen atoms as well. So, we can say that this compound also can undergo a Cannizzaro reaction.
Hence we can conclude that (C) $C{H_3}CHO$ does not undergo Cannizzaro reaction and thus the answer would be: (C) $C{H_3}CHO$.
Note: We should note that the combination of crossed Cannizzaro reaction and aldol condensation is used in the industry to prepare polyols. Polyols are very useful and find many industrial applications. We should note that neopentyl glycol is used in polyesters for resins used in aeroplane or boat manufacturing, varnish coatings, synthetic lubricants, and plasticizers.
Complete step by step solution:
-First of all, we will see what the Cannizzaro reaction is all about.
The Cannizaro reaction is named after Stanislao Cannizzaro when he succeeded in obtaining benzyl alcohol and potassium benzoate from Benzaldehyde in 1853. This reaction involves the simultaneous oxidation and reduction of aldehyde to yield a carboxylic acid and a primary alcohol. Or we can say that Cannizzaro Reaction explains the method to get one molecule of alcohol and one molecule of carboxylic acid from two molecules of a given aldehyde. This reaction involves nucleophilic acyl substitution on an aldehyde molecule and the leaving group will attack another aldehyde.
-We will now talk about how this reaction proceeds or its mechanism. It basically occurs in 3 steps:
Step-1: In this step, the carbonyl group of the aldehyde is attacked by a nucleophile (like the hydroxide ion ($O{H^ - }$)) causing a disproportionation reaction which leads to the formation of an anion which has two negative charges.

Step-2: The dianion formed in the first step will now function as a hydride reducing agent. The dianion is unstable by nature and thus releases a hydride anion which reacts with another aldehyde. This converts the dianion into a carboxylate anion and the aldehyde into an alkoxide anion.

Step-3: In this step, the alkoxide anion gains a proton from water and leads to the formation of alcohol because the alkoxide anion is more basic than water. The carboxylate anion reacts with acid workup to form a carboxylic acid. Here we use an acid workup because carboxylate anion is less basic than water and thus it cannot take a proton from water.

-One of the most important things we should keep in mind about Cannizzaro reaction is that only those compounds undergo Cannizzaro reaction which does not have any alpha hydrogen atoms. Only such compounds will undergo oxidation and reduction or disproportion reaction to yield carboxylic acid and alcohol.
We should know that alpha hydrogen is the hydrogen atom attached to the alpha carbon. And the alpha carbon is the carbon atom present adjacent to the functional group carbon atom.
-We will now observe each option and check who all are capable of undergoing Cannizzaro reaction. We will check them regarding the presence or absence of alpha hydrogens and that aldehyde which does not contain alpha hydrogen will only give Cannizzaro reaction.
For (A):

The above compound is benzaldehyde (${C_6}{H_5}CHO$) and it has no alpha hydrogen atoms, so it will undergo Cannizzaro reaction.
For (B):

The above structure also does not have any alpha hydrogen atoms attached. So, it will also undergo a Cannizzaro reaction.
For (C) $C{H_3}CHO$: The above compound acetaldehyde has 3 alpha hydrogen atoms attached to the alpha carbon. So, from this, we can say that this compound will not undergo a Cannizzaro reaction.
For (D) HCHO: This compound is known as formaldehyde. It also doesn’t have alpha carbon and so no alpha hydrogen atoms as well. So, we can say that this compound also can undergo a Cannizzaro reaction.
Hence we can conclude that (C) $C{H_3}CHO$ does not undergo Cannizzaro reaction and thus the answer would be: (C) $C{H_3}CHO$.
Note: We should note that the combination of crossed Cannizzaro reaction and aldol condensation is used in the industry to prepare polyols. Polyols are very useful and find many industrial applications. We should note that neopentyl glycol is used in polyesters for resins used in aeroplane or boat manufacturing, varnish coatings, synthetic lubricants, and plasticizers.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




