
Aryl fluoride may be prepared from arene diazonium chloride using:
(A) $HB{F_4}/\Delta $
(B) $HB{F_4}/NaN{O_2},Cu$
(C) $CuF/HF$
(D) $Cu/HF$
Answer
233.1k+ views
Hint: Under the presence of nitrous acid, aromatic amines are diazotized. aromatic diazonium salts are considered as one of the intermediates in the preparation of a variety of aromatic compounds including dyes. The Diazonium group is a very good leaving group, so it can be substituted by other groups.
Complete step-by-step answer:We know that aryl fluoride can form by using arene diazonium chloride by using a well known chemistry reaction- Balz-Schiemann reaction. When aryl diazonium chloride is reacted with fluoroboric acid, arene diazonium fluoroborate is precipitated which on further heating decomposes to yield aryl fluoride.
So, by writing the whole reaction we get the following data, which is as follows,
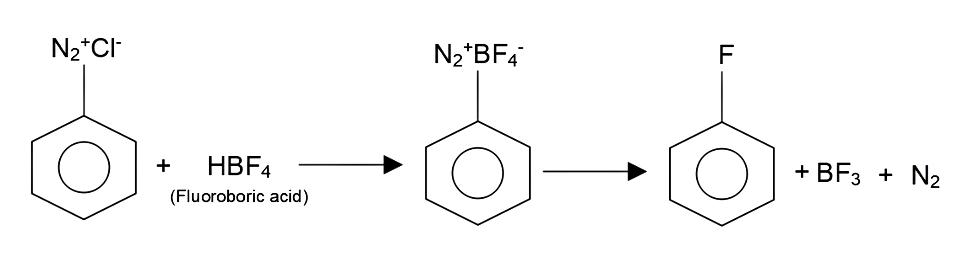
As in the above reaction we can see that when diazonium chloride is reacted as in the presence of $HB{F_4}/\Delta $ it forms the Aryl Fluoride as we see all the steps in the above reaction as with this we get the answer as $HB{F_4}/\Delta $ .
Therefore, the correct answer from this is $HB{F_4}/\Delta $ .
Option ‘A’ is correct
Note: A Point should be noted that Aryl Fluoride forms from Arene diazonium chloride in the presence of $HB{F_4}/\Delta $ . In this reaction the Diazonium group is substituted by the F-group. This is an exothermic reaction as heat is used in this process.
Complete step-by-step answer:We know that aryl fluoride can form by using arene diazonium chloride by using a well known chemistry reaction- Balz-Schiemann reaction. When aryl diazonium chloride is reacted with fluoroboric acid, arene diazonium fluoroborate is precipitated which on further heating decomposes to yield aryl fluoride.
So, by writing the whole reaction we get the following data, which is as follows,

As in the above reaction we can see that when diazonium chloride is reacted as in the presence of $HB{F_4}/\Delta $ it forms the Aryl Fluoride as we see all the steps in the above reaction as with this we get the answer as $HB{F_4}/\Delta $ .
Therefore, the correct answer from this is $HB{F_4}/\Delta $ .
Option ‘A’ is correct
Note: A Point should be noted that Aryl Fluoride forms from Arene diazonium chloride in the presence of $HB{F_4}/\Delta $ . In this reaction the Diazonium group is substituted by the F-group. This is an exothermic reaction as heat is used in this process.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




