
Aniline and methylamine can be differentiated by
(A) Reaction with chloroform and aqueous solution of KOH
(B) Diazotization followed by coupling with phenol
(C) Reaction with $HN{{O}_{2}}$
(D) None of these
Answer
232.8k+ views
Hint: Both the aniline and methyl amine contain an amino group. The difference is that aniline is an aromatic amine, whereas methylamine is an aliphatic amine. To differentiate these compounds, one of the compounds should give a reaction which the others do not.
Complete Step by Step Solution:
The aniline and methyl amine can be differentiated by the diazotization reaction followed by coupling with phenol. The diazotization reaction is the reaction in which an aromatic amine gets converted into its diazonium salt. Also, the coupling reaction is the reaction in which arene diazonium salt reacts with highly reactive aromatic compounds containing electron-donating groups like$OH$, $N{{H}_{2}}$etc. The diazonium salt of aniline, known as benzene diazonium chloride, is prepared by the reaction of aniline with sodium nitrite $(NaN{{O}_{2}})$ in the presence of an excess of mineral acid. This benzene diazonium chloride will then undergo a coupling reaction at a low temperature (273K-278K) with phenol to form hydroxyazobenzene.
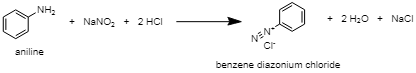
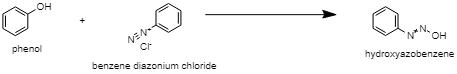
whereas methyl amine does not react with phenols.
Correct Option: (B) Diazotization followed by coupling with phenol.
Additional Information: The benzene diazonium chloride can also react with 2-naphthol to form 2-naphthol aniline dye, which is bright orange in colour. The diazonium salts can be used in the synthesis of various organic compounds. These salts also have applications in nanotechnology and in the dye and pigment industries.
Note: The Benzene diazonium chloride can also react with aniline in the presence of dilute alkali like $NaOH$ in acidic medium to form p-aminoazobenzene, which is also known as yellow dye.
Complete Step by Step Solution:
The aniline and methyl amine can be differentiated by the diazotization reaction followed by coupling with phenol. The diazotization reaction is the reaction in which an aromatic amine gets converted into its diazonium salt. Also, the coupling reaction is the reaction in which arene diazonium salt reacts with highly reactive aromatic compounds containing electron-donating groups like$OH$, $N{{H}_{2}}$etc. The diazonium salt of aniline, known as benzene diazonium chloride, is prepared by the reaction of aniline with sodium nitrite $(NaN{{O}_{2}})$ in the presence of an excess of mineral acid. This benzene diazonium chloride will then undergo a coupling reaction at a low temperature (273K-278K) with phenol to form hydroxyazobenzene.


whereas methyl amine does not react with phenols.
Correct Option: (B) Diazotization followed by coupling with phenol.
Additional Information: The benzene diazonium chloride can also react with 2-naphthol to form 2-naphthol aniline dye, which is bright orange in colour. The diazonium salts can be used in the synthesis of various organic compounds. These salts also have applications in nanotechnology and in the dye and pigment industries.
Note: The Benzene diazonium chloride can also react with aniline in the presence of dilute alkali like $NaOH$ in acidic medium to form p-aminoazobenzene, which is also known as yellow dye.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




