
An organic compound (A) (molecular formula C6H12O2) was hydrolysed with dilute H2SO4 to give a carboxylic acid (B) and alcohol (C). ‘C’ gives white turbidity immediately when treated with anhydrous ZnCl2 and conc. HCl. The organic compound (A) is:
(A)
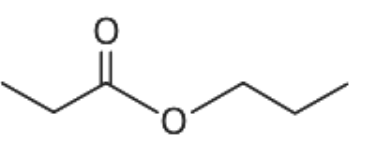
(B)
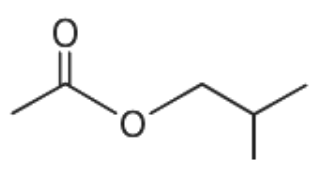
(C)
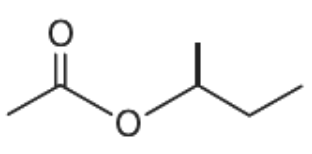
(D)
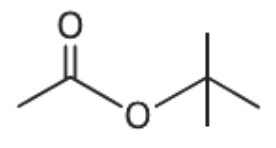
Answer
232.8k+ views
Hint: In the given question anhydrous ZnCl2 and concentrated HCl are given. Both of these together form the Lucas reagent. Now, lucas reagent gives precipitates at different times when reacted with primary, secondary and tertiary alcohols. Only one of the three gives an immediate precipitate with Lucas reagent.
Complete Step by Step Solution:
Anhydrous zinc chloride is dissolved in strong hydrochloric acid to form "Lucas' reagent." The classification of low molecular weight alcohols is done using this method. The chloride substitutes for a hydroxyl group in the process, which is a substitution. A transition from clear and colourless to muddy, signifying the development of a chloroalkane, indicates a positive test.
Alcohols can be distinguished into primary, secondary, and tertiary alcohols using the Lucas test. The three classes of alcohols' varying reactivity with hydrogen halides via an SN1 reaction provide the basis for this:
ROH + HCl → RCl + H2O.
Before the reaction with Lucas Reagent the compound A asked in the question undergoes hydrolysis. On the hydrolysis reaction of the given compounds, one will always be a carboxylic acid whereas the other three will be either primary, secondary or tertiary alcohol.
From the given options, when the compound given in option (A) undergoes hydrolysis it gives secondary alcohol. Similarly, the compound given in option (C) and (D) gives primary alcohol. These primary and secondary alcohols do not give immediate but delayed precipitation with Lucas reagent. Only option (B) gives tertiary alcohol.
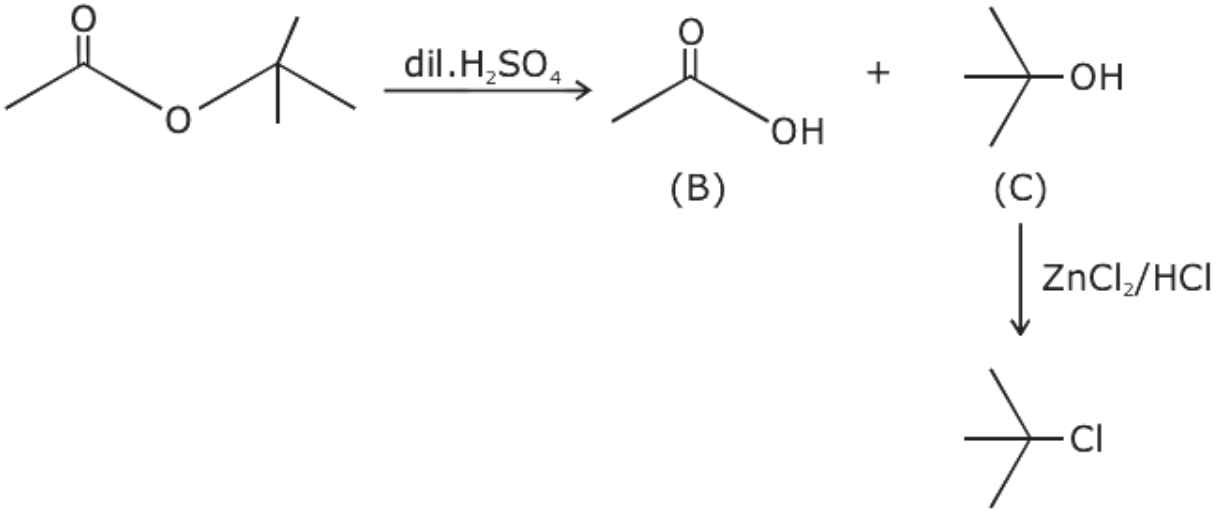
Thus, the alcohol obtained from the compound given in option (B) gives immediate precipitate with Lucas reagent.
Hence, the correct answer is option (B)
Note: The reagent is a combination of concentrated HCl and ZnCl2 in an equimolar ratio. The nucleophile Cl, which is present in excess, rapidly attacks the protonated alcohol, causing the H2O group to depart and form a carbocation, which then yields the chloroalkane. Due to the limited solubility of the organic chloride in the aqueous mixture, tertiary alcohols react with Lucas reagent instantly as shown by the turbidity. Secondary alcohols respond in about five minutes (depending on their solubility). At room temperature, primary alcohols do not significantly react with the Lucas reagent.
Complete Step by Step Solution:
Anhydrous zinc chloride is dissolved in strong hydrochloric acid to form "Lucas' reagent." The classification of low molecular weight alcohols is done using this method. The chloride substitutes for a hydroxyl group in the process, which is a substitution. A transition from clear and colourless to muddy, signifying the development of a chloroalkane, indicates a positive test.
Alcohols can be distinguished into primary, secondary, and tertiary alcohols using the Lucas test. The three classes of alcohols' varying reactivity with hydrogen halides via an SN1 reaction provide the basis for this:
ROH + HCl → RCl + H2O.
Before the reaction with Lucas Reagent the compound A asked in the question undergoes hydrolysis. On the hydrolysis reaction of the given compounds, one will always be a carboxylic acid whereas the other three will be either primary, secondary or tertiary alcohol.
From the given options, when the compound given in option (A) undergoes hydrolysis it gives secondary alcohol. Similarly, the compound given in option (C) and (D) gives primary alcohol. These primary and secondary alcohols do not give immediate but delayed precipitation with Lucas reagent. Only option (B) gives tertiary alcohol.

Thus, the alcohol obtained from the compound given in option (B) gives immediate precipitate with Lucas reagent.
Hence, the correct answer is option (B)
Note: The reagent is a combination of concentrated HCl and ZnCl2 in an equimolar ratio. The nucleophile Cl, which is present in excess, rapidly attacks the protonated alcohol, causing the H2O group to depart and form a carbocation, which then yields the chloroalkane. Due to the limited solubility of the organic chloride in the aqueous mixture, tertiary alcohols react with Lucas reagent instantly as shown by the turbidity. Secondary alcohols respond in about five minutes (depending on their solubility). At room temperature, primary alcohols do not significantly react with the Lucas reagent.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




