
Among the naturally occurring carbohydrates, furanose ring is found in the:
A.glucose unit of cane sugar
B.glucose unit of cellulose
C.fructose unit of cane sugar
D.galactose unit of lactose
Answer
232.8k+ views
Hint: To answer this question, you should recall the concept of furanose sugars. Furanose is a term which designates carbohydrates with a five-membered ring (cyclic) structure, consisting of 4 carbon atoms and single oxygen. It is called furanose because of its structural similarity to furan.
Complete Step by step solution:
We know that the different structures of carbohydrates that result from a keto form are:
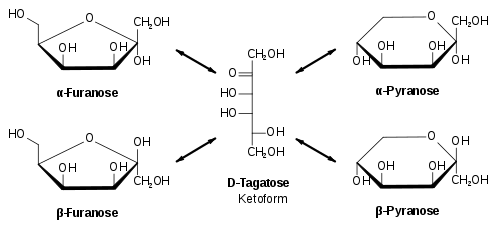
We know that the reducing carbohydrates possess anomeric carbon which acts as a chiral centre for the cyclization of the molecules. The cyclization of the carbohydrate molecules forms two different Structural isomers –
1) Pyranose
2) furanose
The same rule applies to fructose as it also exists in a cyclic form which is known as furanose with an analogy to the compound furan which is a five-membered cyclic compound with one oxygen and four carbon atoms. The structure of furanose can be drawn as:
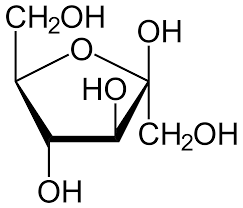
Therefore, we can conclude that the correct answer to this question is option C.
Note: You should know that if you have seen drawings of sugars before, you might not have noticed the carbonyl. The reason is that carbonyl is usually "masked" as a hemiacetal structure. The hemiacetal structure is generated when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. We can see from the structure of fructose that the keto group is present on the second carbon. Now two possibilities for cyclization exist: If attacked by the \[{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{OH}}\] on the 6th carbon it will generate pyranose (6-membered) ring and second that, attacked by the \[{\text{ - CH}}\left( {{\text{OH}}} \right){\text{ - }}\] group on the 5th carbon it will result in a furanose. In general, we can conclude that pyranose rings are more stable than furanose rings, because there are more conformational isomeric structures available for pyranose vs. furanose.
Complete Step by step solution:
We know that the different structures of carbohydrates that result from a keto form are:

We know that the reducing carbohydrates possess anomeric carbon which acts as a chiral centre for the cyclization of the molecules. The cyclization of the carbohydrate molecules forms two different Structural isomers –
1) Pyranose
2) furanose
The same rule applies to fructose as it also exists in a cyclic form which is known as furanose with an analogy to the compound furan which is a five-membered cyclic compound with one oxygen and four carbon atoms. The structure of furanose can be drawn as:

Therefore, we can conclude that the correct answer to this question is option C.
Note: You should know that if you have seen drawings of sugars before, you might not have noticed the carbonyl. The reason is that carbonyl is usually "masked" as a hemiacetal structure. The hemiacetal structure is generated when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. We can see from the structure of fructose that the keto group is present on the second carbon. Now two possibilities for cyclization exist: If attacked by the \[{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{OH}}\] on the 6th carbon it will generate pyranose (6-membered) ring and second that, attacked by the \[{\text{ - CH}}\left( {{\text{OH}}} \right){\text{ - }}\] group on the 5th carbon it will result in a furanose. In general, we can conclude that pyranose rings are more stable than furanose rings, because there are more conformational isomeric structures available for pyranose vs. furanose.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




