
Among the following species : ${\text{N}}{{\text{F}}_{\text{3}}}{\text{,N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{,H}}{{\text{N}}_{\text{3}}}$ , the isostructural pairs are:
(A) $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,N}}{{\text{O}}_{\text{3}}}^{\text{ - }}} \right]$ and $\left[ {{\text{B}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}} \right]$
(B) $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,H}}{{\text{N}}_{\text{3}}}} \right]$ and $\left[ {{\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}} \right]$
(C) $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}} \right]$ and $\left[ {{\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}} \right]$
(D) $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}} \right]$ and $\left[ {{\text{H}}{{\text{N}}_{\text{3}}}{\text{,B}}{{\text{F}}_{\text{3}}}} \right]$
Answer
233.1k+ views
Hint: Isostructural species are those species which have the same shape and shape hybridization. The geometry of molecules is fixed for a particular hybridization but the shapes may vary depending upon the presence or absence of lone pairs. Therefore, the isostructural species can be identified when their exact shapes are known.
Complete step by step answer:
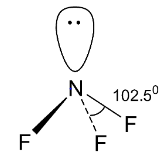
We are given 5 species ${\text{N}}{{\text{F}}_{\text{3}}}{\text{,N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{,H}}{{\text{N}}_{\text{3}}}$ and we need to find out the isostructural pairs from these. Let us at first check the structures of these 5 species. ${\text{N}}{{\text{F}}_{\text{3}}}$ is a trihalide of nitrogen of group 15. Like ammonia, the trihalides of group 15 elemenst also have pyramidal structures. So, ${\text{N}}{{\text{F}}_{\text{3}}}$ will also have a pyramidal structure, i.e., the central element nitrogen is ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. Three of the four ${\text{s}}{{\text{p}}^{\text{3}}}$ orbitals form sigma bonds with fluorine atoms and the fourth ${\text{s}}{{\text{p}}^{\text{3}}}$ orbital contain the lone pair of electrons. The lone pair-bond pair repulsion is greater than the bond pair-bond pair repulsion due to which the expected tetrahedral shape gets distorted and the bond angle becomes less than the tetrahedral bond angle \[109^\circ \] and the structure becomes pyramidal.
${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$ has the following structure.
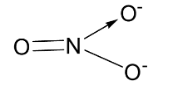
Here, the central element of the molecule which is nitrogen is ${\text{s}}{{\text{p}}^2}$ hybridized. The 3 ${\text{s}}{{\text{p}}^2}$ hybridized orbitals of nitrogen overlaps with one s orbital of oxygen and the nitrogen p orbital makes a double bond with 3 oxygen atoms. Thus, nitrogen is bonded to 3 oxygen atoms and no lone pairs are present and the oxygen atoms lie at the corners of a triangle at the same plane. So the molecule will have slightly bent geometry with bond angle $120^\circ $ and trigonal planar structure.
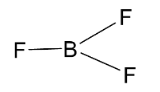
Next, we have ${\text{B}}{{\text{F}}_{\text{3}}}$ molecule which has the following structure.
Here, the central element of the molecule which is boron is ${\text{s}}{{\text{p}}^2}$ hybridized and so the molecule will have a trigonal planar structure. Here, the three ${\text{s}}{{\text{p}}^2}$ hybridized orbitals of boron atoms overlap with p-orbitals of 3 fluorine atoms. All the boron orbitals are arranged in triangular shape and all the atoms lie on the same plane. So, it has a trigonal planar structure with bond angle $120^\circ $ .
Next, the hydronium ion has the following structure.
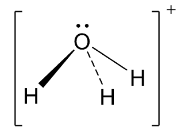
Thus, the central element of the hydronium ion which is oxygen is ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. So, ideally it should have tetrahedral structure. But, in this case, there are 3 bond pairs and one lone pair of electrons which causes distortion leading to pyramidal shape and bond angle becomes $109.5^\circ $.
Lastly, we have hydrazoic acid ${\text{H}}{{\text{N}}_{\text{3}}}$ which is a special hydride of nitrogen. It has the structure as shown below.
Here, nitrogen is sp hybridized and hence hydrazoic acid will have linear structure with bond angle $180^\circ $ .
From the above discussion, we can see that $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}} \right]$ have similar hybridization and structures and $\left[ {{\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}} \right]$ have similar hybridization and structures.
So, the correct option is C.
Note: Isoelectronic species are those species which have the same number of electrons around them. For example, the fluoride ion, neon and sodium ion have 10 electrons around them and so they are isoelectronic. Species which are isoelectronic usually have similar structures and so they are also isostructural. For example, methane and ammonium ion are isoelectronic as well as isostructural as both have tetrahedral structure.
Complete step by step answer:
We are given 5 species ${\text{N}}{{\text{F}}_{\text{3}}}{\text{,N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{,H}}{{\text{N}}_{\text{3}}}$ and we need to find out the isostructural pairs from these. Let us at first check the structures of these 5 species. ${\text{N}}{{\text{F}}_{\text{3}}}$ is a trihalide of nitrogen of group 15. Like ammonia, the trihalides of group 15 elemenst also have pyramidal structures. So, ${\text{N}}{{\text{F}}_{\text{3}}}$ will also have a pyramidal structure, i.e., the central element nitrogen is ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. Three of the four ${\text{s}}{{\text{p}}^{\text{3}}}$ orbitals form sigma bonds with fluorine atoms and the fourth ${\text{s}}{{\text{p}}^{\text{3}}}$ orbital contain the lone pair of electrons. The lone pair-bond pair repulsion is greater than the bond pair-bond pair repulsion due to which the expected tetrahedral shape gets distorted and the bond angle becomes less than the tetrahedral bond angle \[109^\circ \] and the structure becomes pyramidal.

${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$ has the following structure.

Here, the central element of the molecule which is nitrogen is ${\text{s}}{{\text{p}}^2}$ hybridized. The 3 ${\text{s}}{{\text{p}}^2}$ hybridized orbitals of nitrogen overlaps with one s orbital of oxygen and the nitrogen p orbital makes a double bond with 3 oxygen atoms. Thus, nitrogen is bonded to 3 oxygen atoms and no lone pairs are present and the oxygen atoms lie at the corners of a triangle at the same plane. So the molecule will have slightly bent geometry with bond angle $120^\circ $ and trigonal planar structure.

Next, we have ${\text{B}}{{\text{F}}_{\text{3}}}$ molecule which has the following structure.
Here, the central element of the molecule which is boron is ${\text{s}}{{\text{p}}^2}$ hybridized and so the molecule will have a trigonal planar structure. Here, the three ${\text{s}}{{\text{p}}^2}$ hybridized orbitals of boron atoms overlap with p-orbitals of 3 fluorine atoms. All the boron orbitals are arranged in triangular shape and all the atoms lie on the same plane. So, it has a trigonal planar structure with bond angle $120^\circ $ .
Next, the hydronium ion has the following structure.

Thus, the central element of the hydronium ion which is oxygen is ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. So, ideally it should have tetrahedral structure. But, in this case, there are 3 bond pairs and one lone pair of electrons which causes distortion leading to pyramidal shape and bond angle becomes $109.5^\circ $.
Lastly, we have hydrazoic acid ${\text{H}}{{\text{N}}_{\text{3}}}$ which is a special hydride of nitrogen. It has the structure as shown below.
Here, nitrogen is sp hybridized and hence hydrazoic acid will have linear structure with bond angle $180^\circ $ .
From the above discussion, we can see that $\left[ {{\text{N}}{{\text{F}}_{\text{3}}}{\text{,}}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}} \right]$ have similar hybridization and structures and $\left[ {{\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}{\text{,B}}{{\text{F}}_{\text{3}}}} \right]$ have similar hybridization and structures.
So, the correct option is C.
Note: Isoelectronic species are those species which have the same number of electrons around them. For example, the fluoride ion, neon and sodium ion have 10 electrons around them and so they are isoelectronic. Species which are isoelectronic usually have similar structures and so they are also isostructural. For example, methane and ammonium ion are isoelectronic as well as isostructural as both have tetrahedral structure.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




