
Acetaldehyde reacts with ${\text{NaOH}}$ to form:
A. 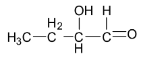
B. 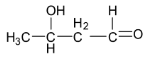
C. 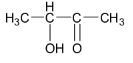
D. 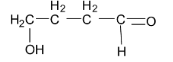
Answer
242.7k+ views
Hint: The carbon atom next to the carbonyl group in an aldehyde or a ketone is called ${{\alpha }}$ - carbon and the hydrogens attached to it are called ${{\alpha }}$ - hydrogens. These ${{\alpha }}$ - hydrogens are acidic due to the electron withdrawing inductive effect of the carbonyl group and so they can be easily abstracted by strong bases to give enolate ions.
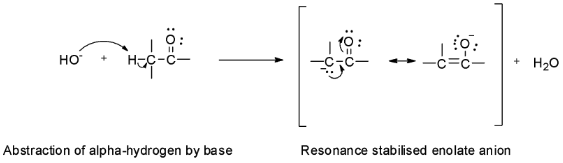
Based on this acidity of ${{\alpha }}$ - hydrogens, the aldol condensation reaction involves the reaction of aldehyde or ketone with dilute alkali to form a ${{\beta }}$ - hydroxyaldehyde or a ${{\beta }}$ - hydroxyketone. These ${{\beta }}$ - hydroxyaldehyde or ${{\beta }}$ - hydroxyketone are called aldols.
Complete step by step answer:
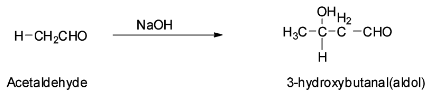
Acetaldehyde is an aldehyde containing ${{\alpha }}$ - hydrogen atoms and so they can be easily abstracted by a base. So acetaldehyde will undergo aldol condensation reaction with sodium hydroxide to give 3- hyroxybutanal which is an aldol. The reaction is shown below:
The mechanism of the above reaction is discussed below.
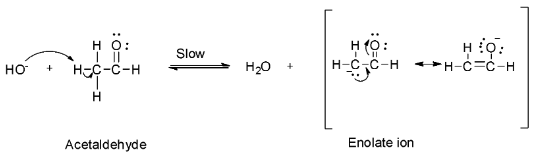
Step 1: Abstraction of acidic alpha hydrogen by the sodium hydroxide base to form an enolate ion.
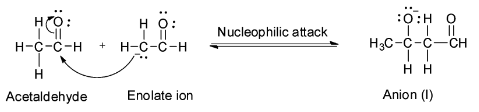
Step 2: Nucleophilic attack of the enolate on the second acetaldehyde molecule to form the anion (I).
Step 3: Abstraction of proton from water by the anion (I) to form aldol.

Option A is not correct as the given aldehyde structure contains hydroxy group in the alpha position and so it is not a betahydroxyaldehyde or aldol.
Option C is not correct as the given ketone structure contains hydroxy group in the alpha position and so it is not a betahydroxyketone or aldol.
Option D is not correct as the given aldehyde structure contains hydroxy group in the gamma position and so it is not a betahydroxyaldehyde or aldol.
Thus, option B is correct.
Note:
Aldehydes which do not have ${{\alpha }}$ - hydrogen atoms will not undergo aldol condensation reaction.
For example, formaldehyde does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give beta hydroxy aldehyde or aldol. Its structure is shown below.
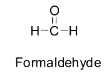
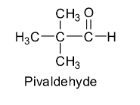
Pivaldehyde also does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give aldol. Its structure is shown below.

Based on this acidity of ${{\alpha }}$ - hydrogens, the aldol condensation reaction involves the reaction of aldehyde or ketone with dilute alkali to form a ${{\beta }}$ - hydroxyaldehyde or a ${{\beta }}$ - hydroxyketone. These ${{\beta }}$ - hydroxyaldehyde or ${{\beta }}$ - hydroxyketone are called aldols.
Complete step by step answer:
Acetaldehyde is an aldehyde containing ${{\alpha }}$ - hydrogen atoms and so they can be easily abstracted by a base. So acetaldehyde will undergo aldol condensation reaction with sodium hydroxide to give 3- hyroxybutanal which is an aldol. The reaction is shown below:

The mechanism of the above reaction is discussed below.
Step 1: Abstraction of acidic alpha hydrogen by the sodium hydroxide base to form an enolate ion.

Step 2: Nucleophilic attack of the enolate on the second acetaldehyde molecule to form the anion (I).

Step 3: Abstraction of proton from water by the anion (I) to form aldol.

Option A is not correct as the given aldehyde structure contains hydroxy group in the alpha position and so it is not a betahydroxyaldehyde or aldol.
Option C is not correct as the given ketone structure contains hydroxy group in the alpha position and so it is not a betahydroxyketone or aldol.
Option D is not correct as the given aldehyde structure contains hydroxy group in the gamma position and so it is not a betahydroxyaldehyde or aldol.
Thus, option B is correct.
Note:
Aldehydes which do not have ${{\alpha }}$ - hydrogen atoms will not undergo aldol condensation reaction.
For example, formaldehyde does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give beta hydroxy aldehyde or aldol. Its structure is shown below.

Pivaldehyde also does not contain any ${{\alpha }}$ - hydrogen atom. So there are no acidic hydrogens available for abstraction by bases and hence it cannot undergo an aldol reaction to give aldol. Its structure is shown below.

Recently Updated Pages
WBJEE 2026 Registration Started: Important Dates Eligibility Syllabus Exam Pattern

Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26




