
A thermally isolated cylindrical closed vessel of height 8m is kept vertically. It is divided into two equal parts by a diathermic (perfect thermal conductor) frictionless partition of mass 8.3kg. Thus the partition is held initially at a distance of 4m from the top, as shown in the schematic figure below. Each of the two parts of the vessel contains 0.1 mole of an ideal gas at temperature 300K. The partition is now released and moves without any gas leaking from one part of the vessel to the other. When equilibrium is reached, the distance of the partition from the top (in m ) will be __________. (Take the acceleration due to gravity=\[10{\rm{ m}}{{\rm{s}}^{ - 2}}\]and the universal gas constant=\[8.3{\rm{ mo}}{{\rm{l}}^{ - 1}}{K^{ - 1}}\])
Answer
232.8k+ views
Hint:A thermodynamic process can be defined as a system that moves from one state to another. Here we use the ideal gas equation also called the general gas equation which gives the behaviour of many gases under many conditions.
Formula used
Ideal gas equation is given as:
\[PV = nRT\]
Where P is pressure, V is volume, n is the amount of substance, R is the universal gas constant and T is temperature.
Complete step by step solution:
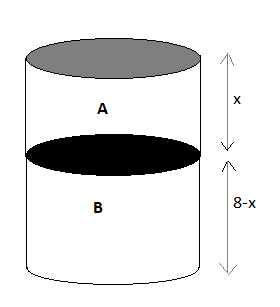
Image: A thermally isolated cylindrical closed vessel.
Let a cylindrical is divided into two equal parts by a diathermic as A and B and the distance of the partition from the top be x.
In each part of the vessel contains, number of mole of an ideal gas, n= 0.1
Temperature, T=300K
For both partition A and B after release is,
As we know ideal gas equation, \[PV = nRT\]
\[{P_A}A \times x = nRT\]
\[\begin{array}{l} \Rightarrow {P_A}(A \times x) = 0.1 \times 8.3 \times 300\\ \Rightarrow {P_A} = \dfrac{{0.1 \times 8.3 \times 300}}{{A \times x}}\end{array}\]
Similarly,
\[\begin{array}{l}{P_B}A \times (x - 8) = 0.1 \times 8.3 \times 300\\ \Rightarrow {P_B} = \dfrac{{0.1 \times 8.3 \times 300}}{{A(x - 8)}}\end{array}\]
At equilibrium condition,
\[{P_A}A + mg = {P_2}A\]
Substituting the values of \[{P_A}{\rm{ }}and{\rm{ }}{P_B}\], we get
\[\dfrac{{0.1 \times 8.3 \times 300}}{{A \times x}} + 8.3 \times 10 = \dfrac{{0.1 \times 8.3 \times 300}}{{A(x - 8)}}\]
\[{x^2} - 2x - 24 = 0\]
On solving
$\therefore x=6$
Hence when equilibrium is reached, the distance of the partition from the top will be 6m.
Note: In thermodynamics, a diathermal wall allows heat to pass through it. Whereas an adiabatic wall does not allow heat to pass through it. For a given thermodynamic process are the movement of heat energy between the systems.
Formula used
Ideal gas equation is given as:
\[PV = nRT\]
Where P is pressure, V is volume, n is the amount of substance, R is the universal gas constant and T is temperature.
Complete step by step solution:

Image: A thermally isolated cylindrical closed vessel.
Let a cylindrical is divided into two equal parts by a diathermic as A and B and the distance of the partition from the top be x.
In each part of the vessel contains, number of mole of an ideal gas, n= 0.1
Temperature, T=300K
For both partition A and B after release is,
As we know ideal gas equation, \[PV = nRT\]
\[{P_A}A \times x = nRT\]
\[\begin{array}{l} \Rightarrow {P_A}(A \times x) = 0.1 \times 8.3 \times 300\\ \Rightarrow {P_A} = \dfrac{{0.1 \times 8.3 \times 300}}{{A \times x}}\end{array}\]
Similarly,
\[\begin{array}{l}{P_B}A \times (x - 8) = 0.1 \times 8.3 \times 300\\ \Rightarrow {P_B} = \dfrac{{0.1 \times 8.3 \times 300}}{{A(x - 8)}}\end{array}\]
At equilibrium condition,
\[{P_A}A + mg = {P_2}A\]
Substituting the values of \[{P_A}{\rm{ }}and{\rm{ }}{P_B}\], we get
\[\dfrac{{0.1 \times 8.3 \times 300}}{{A \times x}} + 8.3 \times 10 = \dfrac{{0.1 \times 8.3 \times 300}}{{A(x - 8)}}\]
\[{x^2} - 2x - 24 = 0\]
On solving
$\therefore x=6$
Hence when equilibrium is reached, the distance of the partition from the top will be 6m.
Note: In thermodynamics, a diathermal wall allows heat to pass through it. Whereas an adiabatic wall does not allow heat to pass through it. For a given thermodynamic process are the movement of heat energy between the systems.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




