
A reaction of \[0.1\] mole of benzyl amine with bromomethane gave \[23g\] of benzyl trimethyl ammonium bromide. The number of moles of bromomethane consumed in this reaction are \[n \times {10^{ - 1}}\] , when \[n = \] _______. (Round off to the Nearest Integer). [Given: Atomic masses: C: \[12.0{\text{ }}u\] , H: \[1.0{\text{ }}u\], N: \[14.0{\text{ }}u\], Br: \[80.0{\text{ }}u\] ]
Answer
233.1k+ views
Hint: The structure of benzyl amine is as follows:
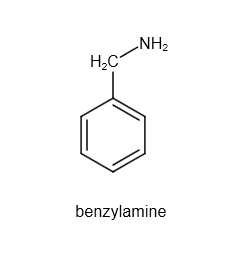
Image: Structure of benzylamine
The structure of benzyl trimethyl ammonium bromide is as follows:
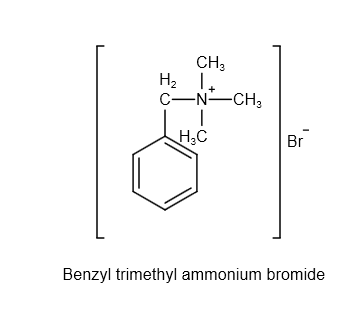
Image: structure of benzyl trimethyl ammonium bromide
Complete Step by Step Solution:
There is a reaction between benzylamine and bromoethane produces benzyl trimethyl ammonium bromide. The balanced chemical reaction is as follows:
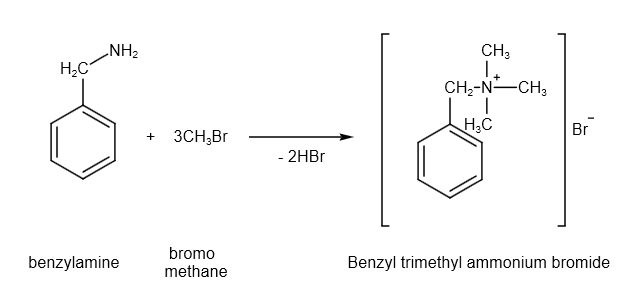
Image: reaction between benzylamine and bromomethane
From the reaction stoichiometry, one mole of benzylamine reacts with three moles of bromomethane and produces one mole of benzyl trimethyl ammonium bromide.
Here, \[0.1\] mole of benzyl amine reacts with bromomethane and gave \[23g\] of benzyl trimethyl ammonium bromide.
First of all, we have to calculate the molar mass of benzyl trimethyl ammonium bromide.
From the structure, the chemical formula of benzyl trimethyl ammonium bromide is \[{C_{10}}{H_{16}}NBr\] .
Calculate the molar mass of \[{C_{10}}{H_{16}}NBr\] from the given atomic masses as follows:
\[
{\text{molar mass of }}{C_{10}}{H_{16}}NBr = 10\left( {12.0} \right) + 16\left( {1.0} \right) + 14.0 + 80.0 \\
\Rightarrow {\text{molar mass of }}{C_{10}}{H_{16}}NBr = 230 \\
\]
Therefore, the molar mass of \[{C_{10}}{H_{16}}NBr\] is \[230\].
We have to calculate the moles of \[{C_{10}}{H_{16}}NBr\] from given mass ( \[23g\] ).
We know that,
\[n = \dfrac{{weight}}{{mol.mass}}\]
Substituting the values,
\[
{n_{{C_{10}}{H_{16}}NBr}} = \dfrac{{23}}{{230}} \\
\Rightarrow {n_{{C_{10}}{H_{16}}NBr}} = 0.1 \\
\]
From the reaction stoichiometry,
\[1\] mole of \[{C_{10}}{H_{16}}NBr\] formed from \[3\] moles of bromomethane.
Calculate the number of moles of bromomethane requires for \[0.1\] mole of \[{C_{10}}{H_{16}}NBr\] formation as follows:
\[
{n_{C{H_3}Br}} = 0.1{\text{ }} \times \dfrac{3}{1} \\
\Rightarrow {n_{C{H_3}Br}} = 0.3 \\
\]
The number of moles consumed in the reaction are \[n \times {10^{ - 1}}\] .
So, we can say that,
\[
n \times {10^{ - 1}} = {n_{C{H_3}Br}} \\
\Rightarrow n \times {10^{ - 1}} = 0.3 \\
\Rightarrow n = 3 \\
\]
Therefore, the value of \[n\] is \[3\] .
Note: Mole, or mol, is a common scientific unit in chemistry that is used to measure vast amounts of tiny objects such as molecules, atoms, or other particles. The number of moles of a solute in a litre of solution is known as molarity. A mole is a unit of measurement that helps us match the particles of a substance to its mass. The molecular weight, also known as molar mass, is the sum of the masses of each atom in grammes that make up a mole of a molecule.

Image: Structure of benzylamine
The structure of benzyl trimethyl ammonium bromide is as follows:

Image: structure of benzyl trimethyl ammonium bromide
Complete Step by Step Solution:
There is a reaction between benzylamine and bromoethane produces benzyl trimethyl ammonium bromide. The balanced chemical reaction is as follows:

Image: reaction between benzylamine and bromomethane
From the reaction stoichiometry, one mole of benzylamine reacts with three moles of bromomethane and produces one mole of benzyl trimethyl ammonium bromide.
Here, \[0.1\] mole of benzyl amine reacts with bromomethane and gave \[23g\] of benzyl trimethyl ammonium bromide.
First of all, we have to calculate the molar mass of benzyl trimethyl ammonium bromide.
From the structure, the chemical formula of benzyl trimethyl ammonium bromide is \[{C_{10}}{H_{16}}NBr\] .
Calculate the molar mass of \[{C_{10}}{H_{16}}NBr\] from the given atomic masses as follows:
\[
{\text{molar mass of }}{C_{10}}{H_{16}}NBr = 10\left( {12.0} \right) + 16\left( {1.0} \right) + 14.0 + 80.0 \\
\Rightarrow {\text{molar mass of }}{C_{10}}{H_{16}}NBr = 230 \\
\]
Therefore, the molar mass of \[{C_{10}}{H_{16}}NBr\] is \[230\].
We have to calculate the moles of \[{C_{10}}{H_{16}}NBr\] from given mass ( \[23g\] ).
We know that,
\[n = \dfrac{{weight}}{{mol.mass}}\]
Substituting the values,
\[
{n_{{C_{10}}{H_{16}}NBr}} = \dfrac{{23}}{{230}} \\
\Rightarrow {n_{{C_{10}}{H_{16}}NBr}} = 0.1 \\
\]
From the reaction stoichiometry,
\[1\] mole of \[{C_{10}}{H_{16}}NBr\] formed from \[3\] moles of bromomethane.
Calculate the number of moles of bromomethane requires for \[0.1\] mole of \[{C_{10}}{H_{16}}NBr\] formation as follows:
\[
{n_{C{H_3}Br}} = 0.1{\text{ }} \times \dfrac{3}{1} \\
\Rightarrow {n_{C{H_3}Br}} = 0.3 \\
\]
The number of moles consumed in the reaction are \[n \times {10^{ - 1}}\] .
So, we can say that,
\[
n \times {10^{ - 1}} = {n_{C{H_3}Br}} \\
\Rightarrow n \times {10^{ - 1}} = 0.3 \\
\Rightarrow n = 3 \\
\]
Therefore, the value of \[n\] is \[3\] .
Note: Mole, or mol, is a common scientific unit in chemistry that is used to measure vast amounts of tiny objects such as molecules, atoms, or other particles. The number of moles of a solute in a litre of solution is known as molarity. A mole is a unit of measurement that helps us match the particles of a substance to its mass. The molecular weight, also known as molar mass, is the sum of the masses of each atom in grammes that make up a mole of a molecule.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




