
A mixture of two organic compounds was treated with sodium metal in ether solution. Isobutane was obtained as a product. The two chlorine compounds are:
A. Methyl chloride and propyl chloride
B. Methyl chloride and ethyl chloride
C. Isopropyl chloride and methyl chloride
D. Isopropyl chloride and ethyl chloride
Answer
233.1k+ views
Hint: Wurtz reaction involves the interaction of two molecules of alkyl halides and sodium metal in presence of dry ether.
Complete Step by Step Solution:
Many of the substitution reactions which do occur when organometallic reagents are used as nucleophiles proceed with only moderate yields. Coupling of haloalkanes in the presence of an active metal is known as the Wurtz reaction. The reaction is usually accomplished by adding sodium metal to an organohalogen compound in an inert solvent. The organosodium compound which forms combines with unreacted haloalkane.
The general equation of Wurtz reaction is as shown below:
.
where, \[{\rm{R}}\]= alkyl group
\[{\rm{X}}\]= halogen atom
When methyl chloride and propyl chloride is used, the product obtained is butane and sodium chloride. Hence, option (A) is incorrect. The reaction is as shown below:
When methyl chloride and ethyl chloride is used, the product obtained is propane and sodium chloride. Hence, option (B) is incorrect. The reaction is as shown below:
When isopropyl chloride and methyl chloride is used, the product obtained is isobutane and sodium chloride. Hence, option (C) is correct. The reaction is as shown below:
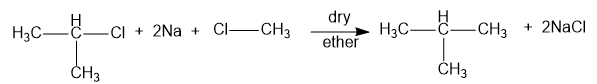
When isopropyl chloride and ethyl chloride is used, the product obtained is isopentane and sodium chloride. Hence, option (D) is incorrect. The reaction is as shown below:
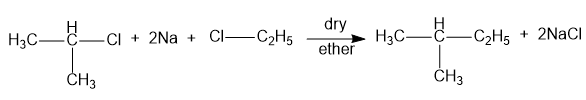
Therefore, option C is correct.
Note: The mechanism for the reaction is believed to involve the formation of nitrogen ylide by the addition of dichlorocarbene that is formed initially. This is followed by the transfer of proton and further elimination of hydrogen chloride to give the desired product.
Complete Step by Step Solution:
Many of the substitution reactions which do occur when organometallic reagents are used as nucleophiles proceed with only moderate yields. Coupling of haloalkanes in the presence of an active metal is known as the Wurtz reaction. The reaction is usually accomplished by adding sodium metal to an organohalogen compound in an inert solvent. The organosodium compound which forms combines with unreacted haloalkane.
The general equation of Wurtz reaction is as shown below:
.
where, \[{\rm{R}}\]= alkyl group
\[{\rm{X}}\]= halogen atom
When methyl chloride and propyl chloride is used, the product obtained is butane and sodium chloride. Hence, option (A) is incorrect. The reaction is as shown below:
When methyl chloride and ethyl chloride is used, the product obtained is propane and sodium chloride. Hence, option (B) is incorrect. The reaction is as shown below:
When isopropyl chloride and methyl chloride is used, the product obtained is isobutane and sodium chloride. Hence, option (C) is correct. The reaction is as shown below:

When isopropyl chloride and ethyl chloride is used, the product obtained is isopentane and sodium chloride. Hence, option (D) is incorrect. The reaction is as shown below:
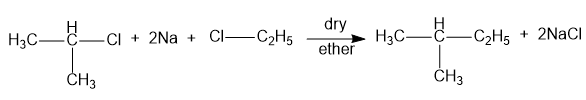
Therefore, option C is correct.
Note: The mechanism for the reaction is believed to involve the formation of nitrogen ylide by the addition of dichlorocarbene that is formed initially. This is followed by the transfer of proton and further elimination of hydrogen chloride to give the desired product.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




