
When a metal is to be extracted from its ore if the gangue associated with the ore, is silica, then
(A) An acidic flux is needed
(B) A basic flux is needed
(C) Both acidic and basic flux are needed
(D) Neither of them is needed
Answer
233.4k+ views
Hint: An ore is a mineral from which pure metal can be extracted using various methods. Gangue are the impurities present in the ore. The flux is a substance which is used to remove the impurities present in the ore. The various materials that can be used as fluxes are silica, lime, dolomite, fluorite, and borax.
Complete Step by Step Answer:
Silica ($Si{{O}_{2}}$) is an acidic impurity. It will combine with a basic flux to form slag. When a metal is separated from its ore, the product left behind is called slag. The slag thus formed is a mixture of metal oxide and silicon dioxide. It occurs as a molten liquid. After cooling, the slag solidifies. Only the formation of the slag, which includes solid impurities and will be settled down in the process of purifying the metal in the metallurgical process, results from the presence of the flux. A basic flux is required when a metal is to be recovered from its ore if the gangue present in the ore is silica.
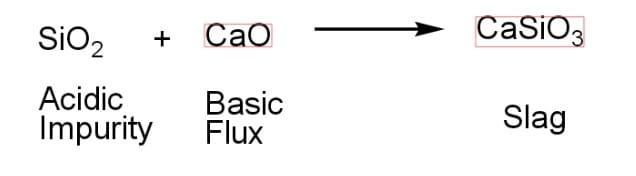
Correct Option: (B) A basic flux is needed.
Note: Flux often has a dual purpose in facilitating the wetting of molten metal by dissolving the oxides already present on the metal surface and serving as an oxygen barrier by covering the hot surface to prevent oxidation.
Complete Step by Step Answer:
Silica ($Si{{O}_{2}}$) is an acidic impurity. It will combine with a basic flux to form slag. When a metal is separated from its ore, the product left behind is called slag. The slag thus formed is a mixture of metal oxide and silicon dioxide. It occurs as a molten liquid. After cooling, the slag solidifies. Only the formation of the slag, which includes solid impurities and will be settled down in the process of purifying the metal in the metallurgical process, results from the presence of the flux. A basic flux is required when a metal is to be recovered from its ore if the gangue present in the ore is silica.

Correct Option: (B) A basic flux is needed.
Note: Flux often has a dual purpose in facilitating the wetting of molten metal by dissolving the oxides already present on the metal surface and serving as an oxygen barrier by covering the hot surface to prevent oxidation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




