




How Does Conduction Work in Everyday Life?
Conduction is a fundamental process of energy transfer occurring within or between materials in direct physical contact. It is observed in various forms, such as the transfer of heat, electrical charge, or sound through suitable conductors. Understanding conduction is essential for solving several topics in physics, especially in advanced exams like JEE.
Definition and Nature of Conduction
Conduction refers to the transfer of energy as heat, electric charge, or vibrational energy through a material without displacement of matter as a whole. This process mainly occurs due to particle interactions within solids and sometimes liquids and gases.
The efficiency and mode of conduction depend on the physical properties of the substance and the type of energy being transferred. Materials that support conduction efficiently are called conductors, while those opposing it are insulators.
Types of Conduction
Three primary types of conduction are significant in physics: thermal conduction, electrical conduction, and sound (acoustic) conduction. Each type involves a distinct mechanism for energy transfer and relies on the material's internal structure.
- Thermal conduction: transfer of heat energy
- Electrical conduction: movement of electric charges
- Sound conduction: propagation of vibrational energy
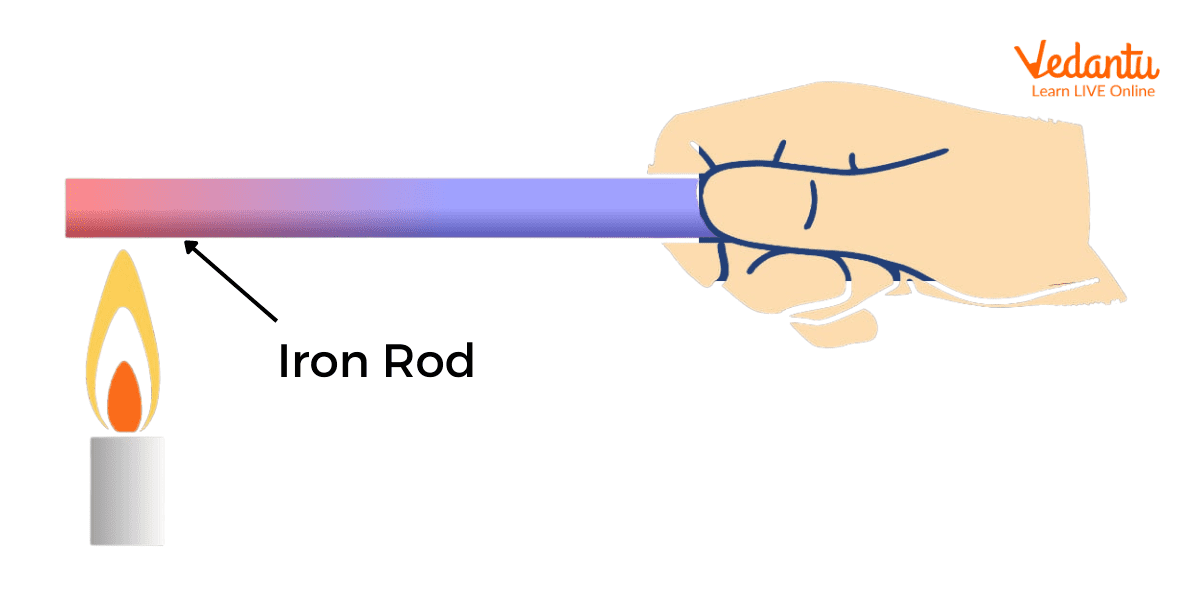
Thermal Conduction
Thermal conduction is the process by which heat energy is transferred within a body or between bodies in physical contact, driven by a temperature gradient. The process occurs without macroscopic movement of the material as a whole.
In solids, especially metals, heat conduction arises from collisions and vibrations of atoms or electrons. The rate of heat flow depends on the material's thermal conductivity and the temperature difference across it.
The quantitative relationship describing thermal conduction is given by Fourier’s Law:
$q = -k \nabla T$
Here, $q$ is the rate of heat transfer per unit area, $k$ is the thermal conductivity, and $\nabla T$ represents the temperature gradient. High thermal conductivity materials such as copper and diamond are effective thermal conductors.
For more on the behavior of materials under heat, see Thermal Expansion Explained.
Electrical Conduction
Electrical conduction is defined as the movement of free electrons or ions within a material under the influence of an electric field. Metals exhibit strong electrical conduction due to the presence of free electrons, while insulators resist the flow of current due to limited charge carriers.
The relationship between current ($I$), voltage ($V$), and resistance ($R$) is given by Ohm’s Law:
$V = IR$
Electrical conductivity ($\sigma$) is the reciprocal of resistivity ($\rho$):
$\sigma = \dfrac{1}{\rho}$
Materials such as copper and silver exhibit high electrical conductivity, making them suitable for use in wires and electrical contacts. Additional information can be found at Electricity And Magnetism Overview.
Sound (Acoustic) Conduction
Sound conduction refers to the transmission of vibrational energy through a material medium, such as solids, liquids, or gases. The propagation of sound relies on the ability of particles to transmit vibrations to neighboring particles.
The speed and efficiency of sound conduction vary with the material's density and elasticity, with solids generally supporting faster transmission compared to gases and liquids.
Mathematical Formulation of Conduction
The calculation of conduction rates depends on the context—thermal, electrical, or acoustic. The most important equations for JEE level are summarized below.
| Concept | Equation |
|---|---|
| Thermal conductivity, $K$ | $K = \dfrac{QL}{A \Delta T}$ |
| Electrical conductivity, $\sigma$ | $\sigma = \dfrac{1}{\rho}$ |
| Fourier’s Law | $q = -k \nabla T$ |
| Ohm's Law | $V = IR$ |
Units Related to Conduction
Thermal conductivity in the SI system is measured in watts per meter per kelvin $(\dfrac{W}{mK})$. Electrical conductivity uses siemens per meter $(\dfrac{S}{m})$. Other units such as $mho/m$ and BTU are also in use for specific applications.
Key Properties of Conduction
- Energy transfer occurs via direct contact
- Particles transfer energy by vibrations or collisions
- No overall movement of material during transfer
- Rate depends on material properties and energy type
- Essential for heat transfer, electrical circuits, and acoustics
Comparison with Other Energy Transfer Mechanisms
Conduction differs from convection and radiation, which are alternative modes of energy transfer. Convection involves bulk movement of fluid, while radiation transmits energy via electromagnetic waves. Comprehensive discussions are provided in Introduction To Thermal Physics.
Common Examples and Applications
- Heat transfer through cooking utensils
- Electric current through metallic wires
- Vibration transmission in solids for soundproofing
- Thermal management in electronic components
- Sound conduction in building materials
Important Laws Associated with Conduction
Fourier’s Law mathematically describes the rate and direction of heat transfer through conduction. Ohm’s Law describes the flow of electric current through a conductor. These laws form the core principles for problem-solving in conduction-related questions.
Summary of Key Points for JEE
- Conduction facilitates energy transfer via direct contact
- Thermal, electrical, and sound conduction have unique features
- Key laws: Fourier’s Law and Ohm’s Law
- Material properties govern conduction efficiency
- Equations are fundamental for numerical solutions
A detailed understanding of conduction supports mastery in related physics topics. For further details, refer to Understanding Conduction and connections to other physics concepts such as Heat Pump Functionality and Energy In Simple Harmonic Motion.
FAQs on What Is Conduction? Understanding Heat Transfer Made Simple
1. What is conduction?
Conduction is the process of heat transfer through a material without the movement of the material itself. In conduction, energy passes from particle to particle via direct contact.
- Occurs in solids, especially metals like copper and aluminum
- Particles vibrate and transfer kinetic energy to neighbors
- No actual movement of matter during the process
- Common in daily life, e.g., a spoon getting hot in a cup of tea
2. Explain how conduction works in metals.
Conduction in metals happens due to free electrons transferring energy rapidly from hot to cold regions.
- Metals have many free electrons, which move and carry heat energy
- When one part is heated, electrons gain kinetic energy and spread it quickly
- Non-metals conduct poorly as they lack free electrons
3. What are the factors affecting the rate of conduction?
The rate of conduction is influenced by several factors, which determine how quickly heat travels through a material.
- Nature of material: Metals conduct faster than non-metals
- Temperature difference: Greater differences increase the rate
- Thickness and length: Thicker or longer materials slow down conduction
- Surface area: Larger area increases heat transfer
4. Give two examples of conduction from daily life.
Common examples of conduction include everyday situations where heat moves through objects by contact.
- A metal pan on the stove heating up from the burner
- A metal spoon warming up in a hot cup of soup
5. What is the difference between conduction, convection, and radiation?
Conduction, convection, and radiation are three different ways of heat transfer.
- Conduction: Heat transfer through direct contact (solids)
- Convection: Heat transfer by movement of fluids (liquids and gases)
- Radiation: Transfer of heat via electromagnetic waves; no medium needed
6. Why are metals good conductors of heat?
Metals are good conductors of heat because they contain free electrons that rapidly transfer energy.
- Free electrons move easily, carrying thermal energy
- Copper, silver, and aluminum are excellent heat conductors
7. What is meant by thermal conductivity?
Thermal conductivity is a material's ability to transfer heat by conduction.
- Measured as the rate at which heat passes through a material
- High thermal conductivity materials (like metals) transfer heat quickly
- Used to compare insulators and conductors
8. What happens to the rate of conduction if the thickness of the material increases?
Increasing the thickness of a material decreases the rate of conduction.
- Heat has to travel a longer distance, slowing down energy transfer
- Materials with greater thickness are better insulators
9. Why is wool a poor conductor of heat?
Wool is a poor conductor of heat because it traps air within its fibers, reducing heat transfer by conduction.
- Air is a good insulator, slowing heat flow
- Woolen clothes keep us warm in winter as they prevent body heat loss
10. What are applications of conduction in daily life?
Conduction is used in many aspects of daily life where effective heat transfer is required.
- Cooking vessels: Metal pans and pots conduct heat from the flame to food
- Irons: Transfer heat to clothes for pressing
- Soldering: Heat conducted to melt metals and join wires
- Radiator pipes: Transfer thermal energy to heat rooms


































