




Differences Between Alpha Helix and Beta Pleated Sheet Structures
In this definition of alpha helix, sheets are characterised through their tight pleats, while in beta pleated sheets, they have looser pleats. Additionally, alpha helix sheets are much less likely to stretch than beta pleated sheets. Alpha-Helix forms all possible hydrogen bonds by twisting into a right handed screw (helix) with the –NH group of each amino acid residue hydrogen bonded to the >C=O of an adjacent turn of the helix.
Beta-pleated sheet structure resembles the pleated folds of drapery.
Helix Meaning
Helix is something spiral in shape that is coiled and contains a repeating pattern. It is a kind of smooth space urve with tangent lines at a constant angle to a fixed axis. Helices are necessary in biology, as the DNA molecule is created as two intertwined helices. Lots of proteins have helical substructures, called alpha helices.
What is Protein?
Protein is a giant, complicated molecule that performs a critical function in our body. It does maximum of the cells' paintings and is needed to shape, feature, and adjust the body’s tissues and organs. Protein is a product of loads or hundreds of long-chain smaller amino acids. The series of amino acids determines the shape and feature of the protein.
Types of Protein
According to their structure, proteins are classified into three types. They are:
Primary Protein: The primary structure of a protein is outlined as the sequence of amino acids connected together to make a polypeptide chain.
Secondary Protein: The next level of protein structure, secondary structure, refers to local folded structures that form inside a polypeptide due to interactions between atoms. The most common forms of secondary structures are the α helix and the and the sheet.
Tertiary Protein: The three-dimensional structure of a polypeptide is termed its tertiary structure.
Primary Protein
The linear sequence of amino acids inside a protein is considered the protein’s primary structure. Each protein features a distinctive primary structure that varies in the pattern amino acids are arranged and the total number of amino acids present within the protein molecule.
Secondary Protein
Secondary protein is often described through intermolecular hydrogen bonds. Alpha-Helices and Beta-Pleated sheets are examples of the secondary shape of the protein.
Alpha-Helix Protein
The most common variety of secondary structure of a protein is the alpha-helix. In the alpha-helix protein, a H bond is created between the N−H group to the C=O group of the amino acid.
The alkyl groups of the alpha-helix chain aren't involved within the H bonds; however, they maintain the alpha-helix structure. Every winding turn in an alpha helix has 3.6 amino acid residues.
Alpha Helix and Beta Pleated Sheet
Alpha-Helix and Beta-Pleated sheets are forms of the secondary shape of the protein. In this arrangement, the polypeptide chains are extended beside one another and then bonded by intermolecular H-bonds. In this structure, all peptide chains are stretched out to almost maximum extension then laid side by side which is held along by intermolecular H bonds. The structure resembles the pleated folds of drapery and thus is known– a beta-pleated sheet.
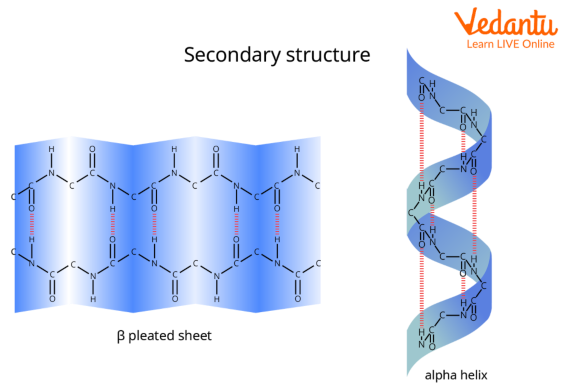
Protein’s Secondary Structure
Beta-Pleated Sheets of Protein
The second important type of secondary form of a protein is the Beta-Pleated Sheets of protein. The beta pleated structure of proteins includes various beta strands connected with the help of using H bonds among adjoining strands. 3 to 10 amino acids are mixed to create a beta-strand polypeptide.
Difference Between Alpha and Beta Helix
The difference between alpha helix and beta sheet on the basis of their definition, shape, formations and bonds are listed in the following table:
Conclusion
Complex proteins have 3 structural organisational levels – primary, secondary, and tertiary. The secondary structures of proteins form the amide chains in several orientations. The peptide chains comprises amino acid sequences bound by amide bonds. Therefore, there are 2 main secondary structures in proteins which are alpha helix and beta helix. This article focuses on the difference between alpha and beta-helix .
FAQs on Alpha Helix and Beta Pleated Sheet: Definitions, Examples & Functions
1. What is an alpha-helix structure in the context of proteins?
An alpha-helix is a common secondary structure found in proteins. It is characterised by a right-handed spiral or coiled shape. This structure is stabilised by intra-chain hydrogen bonds that form between the carbonyl oxygen (C=O) of one amino acid and the amino hydrogen (N-H) of another amino acid located four residues ahead in the same polypeptide chain.
2. What is a beta-pleated sheet structure in proteins?
A beta-pleated sheet, or β-sheet, is another major type of secondary protein structure. It is formed when two or more segments of a polypeptide chain, called beta-strands, line up next to each other. The structure is stabilised by inter-strand hydrogen bonds between the carbonyl oxygen and amino hydrogen groups on adjacent strands, creating a flattened, pleated, sheet-like appearance.
3. What is the main difference in hydrogen bonding between an alpha-helix and a beta-pleated sheet?
The primary difference is the location and arrangement of the hydrogen bonds:
- In an alpha-helix, hydrogen bonds are intra-chain. They form within a single polypeptide chain, connecting amino acids that are close in sequence (the nth and (n+4)th residue).
- In a beta-pleated sheet, hydrogen bonds are inter-strand. They form between different polypeptide chains or between distant segments of the same chain that run alongside each other.
4. What are the two types of beta-pleated sheets, and how do they differ?
The two types of beta-pleated sheets are distinguished by the orientation of their polypeptide strands:
- Parallel β-sheet: In this type, the adjacent beta-strands run in the same direction (e.g., from N-terminus to C-terminus). The hydrogen bonds are evenly spaced but form at an angle.
- Antiparallel β-sheet: Here, the adjacent beta-strands run in opposite directions. This arrangement allows for more stable, linear hydrogen bonds directly opposite each other, making it structurally more stable than the parallel sheet.
5. Who first proposed the alpha-helix and beta-pleated sheet protein structures?
The alpha-helix and beta-pleated sheet structures, which form the basis of protein secondary structure, were first proposed by the scientists Linus Pauling, Robert Corey, and Herman Branson in 1951. Their models were crucial for advancing our understanding of protein biochemistry.
6. Why does an alpha-helix form a coil while a beta-sheet forms a flat sheet?
The distinct shapes arise from their hydrogen bonding patterns. The alpha-helix forms a tight coil because the hydrogen bonds link nearby residues within the same chain, pulling the backbone into a regular, compact spiral. In contrast, the beta-pleated sheet is formed by polypeptide chains that are stretched out and bonded side-by-side, resulting in a more extended, flat, sheet-like conformation.
7. Can a single protein have both alpha-helices and beta-pleated sheets? Provide an example.
Yes, most globular proteins contain a combination of both alpha-helices and beta-pleated sheets, along with other non-regular coils and turns. These secondary structures fold together to create the protein's unique and functional tertiary structure. A good example from the CBSE syllabus is keratin (in hair), which is primarily alpha-helical, while silk fibroin is primarily composed of beta-pleated sheets.
8. How do the R-groups of amino acids influence the formation of these secondary structures?
The R-groups (side chains) of amino acids have a significant impact on secondary structure formation. Their size, charge, and structure can either stabilise or disrupt these patterns. For instance:
- Proline, with its rigid ring structure, is known as a 'helix breaker' because it cannot fit into the regular spiral of an alpha-helix.
- Bulky R-groups or clusters of similarly charged R-groups can cause steric hindrance or electrostatic repulsion, destabilising an alpha-helix.
- Certain sequences of smaller amino acids like glycine and alanine are commonly found in beta-pleated sheets.
9. How do alpha-helices and beta-sheets contribute to a protein's overall tertiary structure?
Alpha-helices and beta-pleated sheets are the foundational local folding patterns (secondary structure) that are then arranged into a more complex, global shape. The tertiary structure is the final three-dimensional arrangement of a single polypeptide chain, which is achieved by the folding and packing of these helices and sheets. This final folding is driven by various interactions between the amino acid R-groups, such as disulphide bridges, hydrophobic interactions, and ionic bonds.
























