NCERT Exemplar for Class 12 Chemistry - General Principles and Processes of Isolation of Elements - Free PDF Download
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 6 - General Principles and Processes of Isolation of Elements solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 6 - General Principles and Processes of Isolation of Elements exercise questions with solutions to help you to revise complete syllabus and score more marks in your Examinations.
NCERT Exemplar is an important resource that students of Class 12 must take good advantage of for their school-level Examinations and even competitive Examinations. There have been a lot of Exams over the past few years where questions that were given in NCERT Exemplar were directly asked in the final Exam as well. It is clear that by getting acquainted with the material given in the NCERT Exemplar, students can score a lot, and that too with very less investment of time.
General Principles and Processes of Isolation of Elements is a theory-based Chapter. With only a few reactions that can be easily memorized by students by regular revision and reviewing of the study material, this is a Chapter that can give them some really easy-to-score marks.
Vedantu brings to you this Free Solutions PDF for NCERT Exemplar Chapter 6 General Principles and Processes of Isolation of Elements with great pleasure. We have made these solutions keeping the exact patterns of several Examinations in mind to ensure that this remains a timeless resource for students studying in Class 12.
Access NCERT Exemplar Solutions for Class 12 Chemistry Chapter 6- General Principles and Processes of Isolation of Elements (Examples, Easy Methods and Step by Step Solutions)
Multiple Choice Questions (Type-I)
1. In the extraction of chlorine by electrolysis of brine
(i) oxidation of $\mathrm{Cl}^{-}$ion to chlorine gas occurs.
(ii) reduction of $\mathrm{Cl}^{-}$ion to chlorine gas occurs.
(iii) For overall reaction $\Delta G^{\circ}$ has a negative value.
(iv) a displacement reaction takes place.
Ans: Option (i)
Explanation: $2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Cl}^{-} \rightarrow 2 \mathrm{OH}^{-}+\mathrm{H}_{2}+\mathrm{Cl}_{2}$
The $\Delta G^{\circ}$ is $+422 \mathrm{~kJ}$ for this reaction. We get $\Delta E^{\circ}=-2.2 \mathrm{~V}$, when we convert it to $\Delta E^{\circ}$ (using $\left.G^{\circ}=E^{\circ} \mathrm{F}\right) .$ It will, of course, require an external e.m.f. greater than $2.2 \mathrm{~V} .$ However, electrolysis necessitates an excess potential in order to overcome some other impeding reactions. Thus, $\mathrm{Cl}_{2}$ is obtained through electrolysis, which produces $\mathrm{H} 2$ and aqueous $\mathrm{NaOH}$ as byproducts. In addition, molten $\mathrm{NaCl}$ is electrolyzed. However, in that case, Na metal is formed rather than $\mathrm{NaOH}$.
2. When copper ore is mixed with silica, in a reverberatory furnace copper matte is produced. The copper matte contains
(i) sulfides of copper (II) and iron (II)
(ii) sulfides of copper (II) and iron (III)
(iii) sulfides of copper (I) and iron (II)
(iv) sulfides of copper (I) and iron (III)
Ans: Option (iii)
Copper ore is mixed with silica before heating in a reverberatory furnace. When heated in a reverberatory furnace, the iron oxide slags of iron silicate and copper are produced. This product is produced in the form of copper matte in the furnace. Presence of $\mathrm{Cu}_{2} \mathrm{~S}$ and $\mathrm{FeS}$ is observed in copper matte. In these compounds, we observe the sulfides of Copper (I) $\mathrm{Cu}^{2+}$ and Iron (II) $\mathrm{Fe}^{2+}$.
3. Which of the following reactions is an example of autoreduction?
(i) $\mathrm{Fe}_{3} \mathrm{O}_{4}+4 \mathrm{CO} \rightarrow 3 \mathrm{Fe}+4 \mathrm{CO}_{2}$
(ii) $\mathrm{Cu}_{2} \mathrm{O}+\mathrm{C} \rightarrow 2 \mathrm{Cu}+\mathrm{CO}$
(iii) $\mathrm{Cu}^{2+}(a q)+\mathrm{Fe}(s) \rightarrow \mathrm{Cu}(s)+\mathrm{Fe}^{2+}(a q)$
(iv) $\mathrm{Cu}_{2} \mathrm{O}+\frac{1}{2} \mathrm{Cu}_{2} \mathrm{~S} \rightarrow 3 \mathrm{Cu}+\frac{1}{2} \mathrm{SO}_{2}$
Ans: Option (iv)
Copper (I) oxide is reduced by copper (I) sulfide in this reaction. Because copper is reduced by itself in this process, it is referred to as auto reduction.
4. A number of elements are available in earth's crust but most abundant elements are
(i) $\quad \mathrm{Al}$ and $\mathrm{Fe}$
(ii) $\quad \mathrm{Al}$ and $\mathrm{Cu}$
(iii) $\mathrm{Fe}$ and $\mathrm{Cu}$
(iv) $\quad \mathrm{Cu}$ and $\mathrm{Ag}$
Ans: Option (i)
Explanation: Aluminum is the third most abundant metal in the earth's crust (8.3 percent approx. by weight). It is found in a variety of igneous minerals, including mica and clays. The second most abundant metal in the earth's crust is iron.
5. Zone refining is based on the principle that
(i) impurities of low boiling metals can be separated by distillation.
(ii) impurities are more soluble in molten metal than in solid metal.
(iii) different components of a mixture are differently adsorbed on an adsorbent.
(iv) Vapors of volatile compounds can be decomposed in pure metal.
Ans: Option (ii)
Purification of metal crystals by making a thin region of crystal undergo melting is known as Zone refining. The molten crystal is then moved up along the crystal to get pure form of it. This process is used to get pure form of Silicon and Germanium. Basic principle of the zone refining process is that the impurities are more soluble in molten metal than solid metal to get pure form of metal.
6. In the extraction of copper from its sulfide ore, the metal is formed by the reduction of $\mathrm{Cu}_{2} \mathrm{O}$ with
(i) $\mathrm{FeS}$
(ii) $\mathrm{CO}$
(iii) $\mathrm{Cu}_{2} \mathrm{~S}$
(iv) $\mathrm{SO}_{2}$
Ans: Option (iii)
Reaction takes place as $\mathrm{Cu}_{2} \mathrm{O}+\frac{1}{2} \mathrm{Cu}_{2} \mathrm{~S} \rightarrow 3 \mathrm{Cu}+\frac{1}{2} \mathrm{SO}_{2}$ with a product as bristle copper. This type of reaction is called an auto-reduction reaction as copper is reducing itself with help of other copper compounds.
7. Brine is electrolysed by using inert electrodes. The reaction at anode is (i) $\mathrm{Cl}^{-}(a q) \rightarrow \frac{1}{2} \mathrm{Cl}_{2}(g)+\mathrm{e}^{-} ; E_{\text {cell }}^{\circ}=1.36 \mathrm{~V}$
(ii) $\quad 2 \mathrm{H}_{2} \mathrm{O}(l) \rightarrow \mathrm{O}_{2}(g)+4 \mathrm{H}^{+}+4 e^{-}, E_{\text {cel }}^{\mathrm{ot}}=1.23 \mathrm{~V}$
(iii) $\mathrm{Na}^{+}(a q .)+e \rightarrow \mathrm{Na}(s) ; E_{\text {cell }}^{0}=2.71 \mathrm{~V}$
(iv) $\mathrm{H}^{+}(a q .)+e^{-} \rightarrow \frac{1}{2} \mathrm{H}_{2}(g) ; E_{\text {cell }}^{\circ}=0.00 \mathrm{~V}$
Ans: Correct option (i)
The reaction at the anode with a lower $E^{\circ}$ value is preferred, but oxygen cannot be obtained in this process due to overvoltage.
8. In the metallurgy of aluminum
(i) $\mathrm{Al}^{3+}$ is oxidized to $\mathrm{Al}$ (s).
(ii) graphite anode is oxidized to carbon monoxide and carbon dioxide.
(iii) oxidation state of oxygen changes in the reaction at anode.
(iv) oxidation state of oxygen changes in the overall reaction involved in the process.
Ans: Option (ii)
Reaction involved in the metallurgy of aluminum is $2 \mathrm{Al}_{2} \mathrm{O}_{3}+3 \mathrm{C} \rightarrow 4 \mathrm{Al}+3 \mathrm{CO}_{2}$.
The reaction at cathode is $\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}$
The reaction at anode is
$\mathrm{C}+\frac{1}{2} \mathrm{O}_{2} \rightarrow \mathrm{CO}+2 \mathrm{e} \text { and } \mathrm{C}+\mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+4 \mathrm{e}^{-}$
Hence, from the reaction graphite anode is oxidized to carbon monoxide and carbon dioxide.
9. Electrolytic refining is used to purify which of the following metals?}
(i) $\quad \mathrm{Cu}$ and $\mathrm{Zn}$
(ii) Ge and Si
(iii) $\mathrm{Zr}$ and $\mathrm{Ti}$
(iv) $\mathrm{Zn}$ and $\mathrm{Hg}$
Ans: Option (i)
The electrolytic method can be used to purify zinc and copper. The impure metal is used as an anode in this method. As the cathode, a pure strip of the same metal is used. They are immersed in an appropriate electrolytic bath containing a soluble salt of the same metal.
The more basic metals remain in the solution, while the less basic metals are transferred to the anode mud.
10. Extraction of gold and silver involves leaching the metal with $\mathrm{CN}^{-}$ion. The metal is recovered by
(i) displacement of metal by some other metal from the complex ion.
(ii) Roasting of metal complexes.
(iii) calcination followed by roasting.
(iv) thermal decomposition of metal complexes.
Ans: Option (i)
The cyanide process involves 3 steps:
first step - The finely grounded ore of gold and silver are made to come in contact with the solution containing the cyanide,
Second step - it involves separation of gold and silver from the cyanide solution third step - it involves the recovery of gold and silver in their pure forms precipitating the remaining solution with zinc dust.
Thus, the metal is recovered by displacing $\mathrm{Zn}$ with the metal (Au or Ag) from metal ions.
Note : Answer the questions 11-13 on the basis of Fig. 6.1.
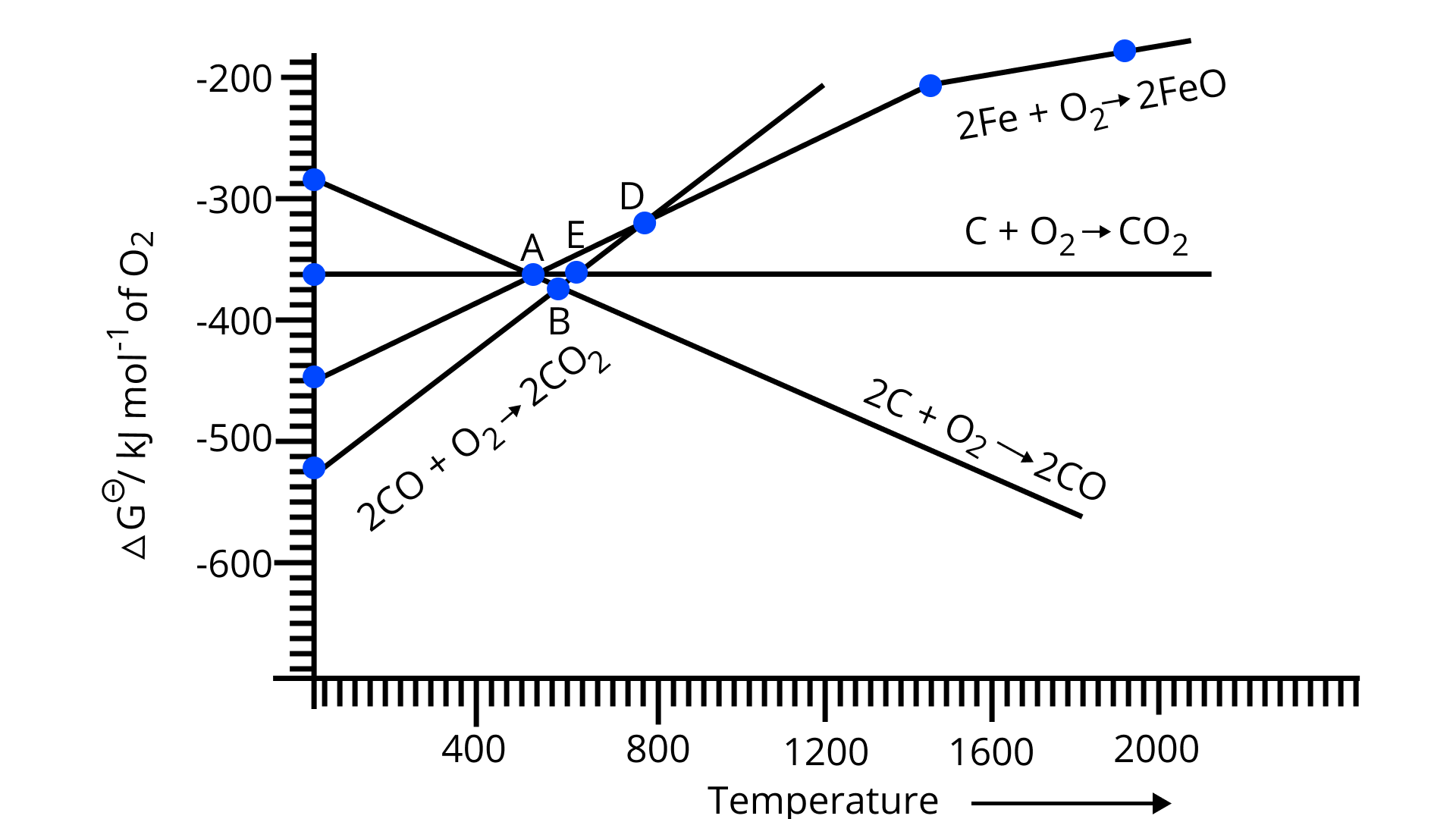
11. Choose the correct option of temperature at which carbon reduces $\mathrm{FeO}$ to iron and produces $\mathrm{CO}$
(i) Below temperature at point $A$.
(ii) Approximately at the temperature corresponding to point $A$.
(iii) Above temperature at point $A$ but below the temperature at point $D$.
(iv) Above temperature at point $A$.
Ans: Option (iv)
Above point $\mathrm{A},\Delta G_{(C, C O)}<\Delta G_{(\mathrm{Fe}, \mathrm{FeO})}$. Thus, carbon reduces $\mathrm{FeO}$ to iron i.e. $\mathrm{Fe}$ and in order carbon is oxidized to carbon monoxide i.e. $\mathrm{CO}$. Both these lines intersect at Point A. As we can see in the figure above point $A$ only carbon could reduce $\mathrm{FeO}$ in $\mathrm{Fe}$ and $\mathrm{CO}$ is being produced.
12. Below point ' $\mathrm{A}$ ' $\mathrm{FeO}$ can
(i) be reduced by carbon monoxide only.
(ii) be reduced by both carbon monoxide and carbon.
(iii) be reduced by carbon only.
(iv) not be reduced by both carbon and carbon monoxide.
Ans: Option (i)
Below point A, only the value of $\Delta G_{\left(\mathrm{CO}, \mathrm{CO}_{2}\right)}$ is less than the value of $\Delta G_{(\mathrm{Fe}, \mathrm{FeO})}$ at the corresponding temperatures. Thus, only carbon monoxide will be able to reduce $\mathrm{FeO}$ to $\mathrm{Fe}$ and will get itself oxidized into $\mathrm{CO}_{2}$.
13. For the reduction of $\mathrm{FeO}$ at the temperature corresponding to point $\mathrm{D}$, which of the following statements is correct?
(i) $\Delta G$ value for the overall reduction reaction with carbon monoxide is zero.
(ii) $\Delta G$ value for the overall reduction reaction with a mixture of 1 mol carbon and $1 \mathrm{~mol}$ oxygen is positive.
(iii) $\Delta G$ value for the overall reduction reaction with a mixture of 2 mol carbon and $1 \mathrm{~mol}$ oxygen will be positive.
(iv) $\Delta G$ value for the overall reduction reaction with carbon monoxide is negative.
Ans: Option (i)
According to the above graph, at point D the equivalent value of $\Delta G$ for the reduction of $\mathrm{FeO}$ is approximately 330 units for the particular temperature. Also, for $\mathrm{CO}$ the equivalent value of $\Delta G$ at the particular temperature for point $D$ is around $-330$ units. Thus, if we calculate the overall value of $\Delta G$ for the reduction of $\mathrm{FeO}$ with carbon monoxide then it is almost zero at point $\mathrm{D}$.
Multiple Choice Questions (Type II)
Note: In the following questions two or more options may be correct.
14. At the temperature corresponding to which of the points in Fig.6.1, $\mathrm{FeO}$ will be reduced to Fe by coupling the reaction $2 \mathrm{FeO} \rightarrow 2 \mathrm{Fe}+\mathrm{O}_{2}$ with all of the following reactions?
(a) $\mathrm{C}+\mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}$ (b) $2 \mathrm{C}+\mathrm{O}_{2} \rightarrow 2 \mathrm{CO}$ and (c) $2 \mathrm{CO}+\mathrm{O}_{2} \rightarrow 2 \mathrm{CO}_{2}$.
(i) Point A
(ii) Point B
(iii) Point D
(iv) Point E
Ans: Option (ii) and (iv)
From Fig.6.1, at point B, all three lines, i.e. for the lines for all three given reactions, are below that of the line of reduction of $\mathrm{FeO}$. This means, that the value of $\Delta G$ for the equivalent temperature at point $\mathrm{B}$ for reduction of $\mathrm{FeO}$ is greater than (positive) that of values of $\Delta G$ for all three reactions (negative). Thus, $\mathrm{FeO}$ will get reduced by all three reactions at point $\mathrm{B}$. Same is the case with point $\mathrm{E}$ as that of point $\mathrm{B}$, the value of $\Delta G$ for reduction of $\mathrm{FeO}$ at $\mathrm{E}$ is greater than the value of $\Delta G$ for all three reactions at the equivalent temperature. Thus, $\mathrm{FeO}$ will get reduced by all three reactions at point $E$.
15. Which of the following options are correct?
(i) Cast iron is obtained by smelting pig iron with scrap iron and coke using hot air blast.
(ii) In extraction of silver, silver is extracted as a cationic complex.
(iii) Nickel is purified by zone refining.
(iv) $\mathrm{Zr}$ and $\mathrm{Ti}$ are purified by van Arkel method.
Ans: Option (i) and (iv)
Pig iron is the iron obtained from the blast that has many impurities (Mn, P, Si, etc) and approx. 4% carbon content. When it is re-melted or cast with the help of scrap iron, then it has less carbon content i.e. $3 \%$ as compared to pig iron.
The $Z r$ and Ti are purified by the help of Arkel Method that contain the following reaction: $\mathrm{Zr}(\mathrm{s})+2 \mathrm{I}_{2} \stackrel{870 \mathrm{k}}{\longrightarrow} \mathrm{ZrI}_{4}(\mathrm{~g}) \underset{\text { tungsten filament }}{\longrightarrow} \underset{\text { Pure }}{\mathrm{Zr}}(s)+2 \mathrm{I}_{2}$
$\mathrm{Ti}+2 \mathrm{I}_{2} \stackrel{530 \mathrm{k}}{\longrightarrow} \mathrm{TiI}_{4} \stackrel{1700 \mathrm{k}}{\longrightarrow} \mathrm{Ti}+2 \mathrm{I}_{2}$
Here we obtain $\mathrm{Zr}$ and Ti as a pure form of solid when purified using the Arkel method.
16. In the extraction of aluminum by Hall-Heroult process, purified $\mathrm{Al}_{2} \mathrm{O}_{3}$ is mixed with $\mathrm{CaF}_{2}$ to
(i) lower the melting point of $\mathrm{Al}_{2} \mathrm{O}_{3}$.
(ii) increase the conductivity of molten mixture.
(iii) reduce $\mathrm{Al}^{3+}$ into $\mathrm{Al}(\mathrm{s})$.
(iv) acts as a catalyst.
Ans: Option (i) and (ii)
In the extraction of alumina by Hall-Heroult's process, alumina is mixed with cryolite with the mixture of fluorspar \& fluoride that increases the conductivity of electrical and lowers the melting point of $\mathrm{Al}_{2} \mathrm{O}_{3}$ Hence option (i) and option (ii) are correct.
17. Which of the following statements is correct about the role of substances added in the froth floatation process?
(i) Collectors enhance the non-wettability of the mineral particles.
(ii) Collectors enhance the wettability of gangue particles.
(iii) By using depressants in the process two sulfide ores can be separated.
(iv) Froth stabilizers decrease wettability of the gangue.
Ans: Option (i) and (iii)
A suspension of powdered ore is made with water during the froth floatation process. Collectors and froth stabilizers are added to it. Collectors (e.g., pine oils, fatty acids, xanthates, etc.) improve the non wettability of the mineral particles, while froth stabilizers (e.g., cresols, aniline) keep the froth stable
18. In the Froth Floatation process, zinc sulfide and lead sulfide can be separated by
(i) using collectors.
(ii) adjusting the proportion of oil to water.
(iii) using depression.
(iv) using froth stabilizers.
Ans: Option (ii) and (iii)
It is possible to separate two sulfide ores using the froth floatation process by adjusting the proportion of oil to water or by using 'depressants.' In the case of an ore containing $\mathrm{ZnS}$ and PbS, for example, the depressant used is NaCN.
19. Common impurities present in bauxite are
(i) $\mathrm{CuO}$
(ii) $\quad \mathrm{ZnO}$
(iii) $\mathrm{Fe}_{2} \mathrm{O}_{3}$
(iv) $\mathrm{SiO}_{2}$
Ans: Option (iii) and (iv)
Bauxite consists of many impurities like hematite, goethite, $\mathrm{Fe}_{2} \mathrm{O}_{3}$, the sand $\mathrm{SiO}_{2}$, etc.'
20. Which of the following ores are concentrated by froth floatation?
(i) Haematite
(ii) Galena
(iii) Copper pyrites
(iv) Magnetite
Ans: Option (ii) and (iii)
The froth floatation method is most commonly used for sulfide ore. Sulfide ores galena (PbS) and copper pyrites $\left(\mathrm{CuFeS}_{2}\right)$ are found here.
21. Which of the following reactions occur during calcination?}
(i) $\quad \mathrm{CaCO}_{3} \rightarrow \mathrm{CaO}+\mathrm{CO}_{2}$
(ii) $2 \mathrm{FeS}_{2}+\frac{11}{2} \mathrm{O}_{2} \rightarrow \mathrm{Fe}_{2} \mathrm{O}_{3}+4 \mathrm{SO}_{2}$
(iii) $\mathrm{Al}_{2} \mathrm{O}_{3} \cdot x \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Al}_{2} \mathrm{O}_{3}+x \mathrm{H}_{2} \mathrm{O}$
(iv) $\mathrm{ZnS}+\frac{3}{2} \mathrm{O}_{2} \rightarrow \mathrm{ZnO}+\mathrm{SO}_{2}$
Ans: Option (i) and (iii)
Calcination is the process of heating when the volatile matter escapes, leaving the metal oxide behind. It is usually done in the absence of air.
22. For the metallurgical process of which of the ores calcined ore can be reduced by carbon?
(i) haematite
(ii) calamine
(iii) iron pyrites
(iv) sphalerite
Ans: Option (i) and (iii)
Explanation: Haematite is an ore of iron that can be calcined and reduced by carbon. In the metallurgical process, calamine ore is calcined ore that can be reduced by carbon. Hence, option (i) and (ii) are correct.
23. The main reactions occurring in blast furnaces during extraction of iron from haematite are
(i) $\quad \mathrm{Fe}_{2} \mathrm{O}_{3}+3 \mathrm{CO} \rightarrow 2 \mathrm{Fe}+3 \mathrm{CO}_{2}$
(ii) $\quad \mathrm{FeO}+\mathrm{SiO}_{2} \rightarrow \mathrm{FeSiO}_{3}$
(iii) $\mathrm{Fe}_{2} \mathrm{O}_{3}+3 \mathrm{C} \rightarrow 2 \mathrm{Fe}+3 \mathrm{CO}$
(iv) $\mathrm{CaO}+\mathrm{SiO}_{2} \rightarrow \mathrm{CaSiO}_{3}$
Ans: Option (i) and (iv)
(i) Carbon monoxide is the primary reducing agent in the furnace.
(ii) This is an endothermic reaction in which heat is absorbed from the furnace. As a result, it is critical not to add too much limestone, as this will cool the furnace. Calcium oxide is a basic oxide that reacts with acidic oxides in the rock, such as silicon dioxide. Calcium silicate is formed when calcium oxide reacts with silicon dioxide.
24. In which of the following methods of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(i) heating with a stream of carbon monoxide.
(ii) heating with iodine.
(iii) liquation.
(iv) distillation.
Ans: Option (i) and (ii)
In the vapor phase refining method, the metal is converted into its volatile compound and then is collected elsewhere. This involves two techniques:
1. Mond Process for refining Nickel: in this process, a volatile complex, nickel tetracarbonyl is formed when nickel is heated with a stream of carbon monoxide.
2. van Arkel Method for refining Zirconium or Titanium: this method is basically used for removal of oxygen and nitrogen present as impurities in the $\mathrm{Zr}$ or Ti metal. These are heated in an evacuated metal with iodine. As iodine is more covalent than these metals, it volatilizes.
25. Which of the following statements are correct?
(i) A depressant prevents a certain type of particle from coming to the froth.
(ii) Copper matte contains $\mathrm{Cu}_{2} \mathrm{~S}$ and $\mathrm{ZnS}$.
(iii) The solidified copper obtained from the reverberatory furnace has a blistered appearance due to evolution of $\mathrm{SO}_{2}$ during the extraction.
(iv) Zinc can be extracted by self-reduction.
Ans: Option (i) and (ii)
Explanation: Depressants are materials that are added for the separation of ores that prevent certain types of particles from coming to froth and forming bubbles. For example, an ore containing ZnS and PbS, NaCN is used as a depressant.
When sulfur ores are blown in hot air along with silica, the solidified metal which is obtained has a blistered appearance due to $\mathrm{SO}_{2}$ evolution.
26. In the extraction of chlorine from brine
(i) $\Delta G^{\circ}$ for the overall reaction is negative.
(ii) $\Delta G^{\circ}$ for the overall reaction is positive.
(iii) $\quad E^{\circ}$ for overall reaction has negative value.
(iv) $\quad E^{\circ}$ for overall reaction has positive value.
Ans: Option (ii) and (iii)
Explanation: Using oxidation method for extraction of chlorine from brine. The reactions involved are:
$2 \mathrm{Cl}^{-}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{OH}^{-}+\mathrm{H}_{2}+\mathrm{Cl}_{2}$
For this reaction, the value of $\Delta G^{\circ}=+422 \mathrm{~kJ}$, which is positive. Using the formula $\Delta G^{\circ}=-n E^{\circ} F$, we get a negative value of $E^{\circ}=-2.2 \mathrm{~V}$.
Short Answer Type
27. Why is an external emf of more than $2.2 \mathrm{~V}$ required for the extraction of $\mathrm{Cl}_{2}$ from the brine?
Ans: Extraction of chlorine from brine is an oxidation method. The reactions involved are:
$2 \mathrm{Cl}^{-}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{OH}^{-}+\mathrm{H}_{2}+\mathrm{Cl}_{2}$
For this reaction, the value of $\Delta G^{\circ}=+422 \mathrm{~kJ}$, which is positive. Using the formula, $\Delta G^{\circ}=-n E^{\circ} F$, we get a negative value of $E^{\circ}=-2.2 \mathrm{~V}$.
Since, $E^{\circ}=-2.2 \mathrm{~V}$, it would naturally require an external voltage greater than $2.2 \mathrm{~V}$ for the reaction to occur.
28. At temperatures above 1073K coke can be used to reduce FeO to Fe. How can you justify this reduction with the Ellingham diagram?
Ans: From Ellingham diagram, we know that $\Delta G_{(\mathrm{C}, \mathrm{CO})}^{\circ}<\Delta G_{(\mathrm{Fe,Fe})}^{\circ}$, the following reactions are:
$\mathrm{C}+\frac{1}{2} \mathrm{O}_{2} \rightarrow \mathrm{CO}$
$2 \mathrm{Fe}+\mathrm{O}_{2} \rightarrow 2 \mathrm{FeO}$
Therefore, FeO can be reduced to Fe by coke.
29. Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulfur, silicon and phosphorus be removed from cast iron?
Ans: Iron oxides are mixed with limestone and coke in order to decompose carbonates and oxidize sulfides. This mixture is then fed into the blast furnace. Many reactions take place in different temperature ranges of the blast furnace. By oxidizing impurities from cast iron in a reverberatory furnace which is lined by hematite, wrought iron or malleable iron is obtained. This is the purest form of commercial iron. This can be shown with the following reaction:
$\mathrm{Fe}_{2} \mathrm{O}_{3}+3 \mathrm{C} \rightarrow 2 \mathrm{Fe}+3 \mathrm{CO}$.
Sulfur, silicone and phosphorus are oxidized and passed into the slag and removed as impurities when limestone is added as a flux to it.
30. How is copper extracted from low grade copper ores?
Ans: Low-grade copper ores contain a small percentage of copper that is $0.27 \%$ copper. By hydrometallurgy, low-grade copper is leached by treating with acid when copper metal goes into solution as copper ions. The solution containing copper ions is treated with scrap iron or hydrogen gas. Since iron or hydrogen is more reactive than copper so they reduced copper ions from solution to copper metal.
31. Write two basic requirements for refining of a metal by Mond process and by Van Arkel Method.
Ans: Both processes have two basic requirements:
(i) The metal should react with an available reagent to form a volatile compound.
(ii) The volatile compound should be easily decomposable, allowing for easy metal recovery.
32. Although carbon and hydrogen are better reducing agents, they are not used to reduce metallic oxides at high temperatures. Why?
Ans: The reduction is the removal of oxygen. Hydrogen and carbon both form oxides that are easy to remove both water and carbon dioxide which are produced as a gas at hot reactions. Although carbon and hydrogen are better reducing agents, they are not used to reduce metallic oxides at high temperatures because at high temperatures carbon and hydrogen react with metals to form carbide and hydrides respectively.
33. How do we separate two sulfide ores by the Froth Floatation Method? Explain with an example.
Ans: Separation of two sulfide ores can be accomplished by adjusting the proportion of oil to water or by using depressants. In the case of an ore containing $\mathrm{ZnS}$ and PbS, for example, the depressant NaCN is used. It forms a complex with $\mathrm{ZnS}$ and prevents it from coming into contact with froth, but PbS remains in contact with froth.
34. The purest form of iron is prepared by oxidizing impurities from cast iron in a reverberatory furnace. Which iron ore is used to line the furnace? Explain by giving a reaction.
Ans: A metal is called Pure when it contains the highest concentration of itself and the least concentration of impurities. While refining of metal, these impurities are removed by various processes aided by the use of other compounds.
Hematite supplies oxygen and oxidizes carbon, silicon, manganese, and phosphorus present in the cast iron to carbon monoxide, manganese oxide, phosphorus pentoxide respectively.
$\mathrm{Fe}_{2} \mathrm{O}_{3}+3 \mathrm{C} \stackrel{\Delta}{\longrightarrow} 2 \mathrm{Fe}+3 \mathrm{CO}$
$3 \mathrm{Si}+2 \mathrm{Fe}_{2} \mathrm{O}_{3} \rightarrow 4 \mathrm{Fe}+3 \mathrm{SiO}_{2}$
$3 \mathrm{~S}+2 \mathrm{Fe}_{2} \mathrm{O}_{3} \rightarrow 3 \mathrm{SO}_{2}+4 \mathrm{Fe}$
Wrought iron is called the purest form of iron as it contains the highest percentage of Iron and the least impurities. It is prepared from cast iron by oxidizing impurities in a reverberatory furnace lined with Hematite.
35. The mixture of compounds $A$ and $B$ is passed through a column of $A l_{2} O_{3}$ by using alcohol as eluent. Compound $A$ is eluted in preference to compound $B$. Which of the compounds $A$ or $B$, is more readily adsorbed on the column?
Ans: Column chromatography is a technique used to separate mixtures based on differences in their affinity towards the stationary phase. If one molecule in a solute has more affinity towards the stationary phase, it would form bonds with the stationary phase and hence remain in the column, Whereas, if the other one has less affinity, it would not form bonds and hence will travel down and come out of the column faster.
As compound $A$ is eluted in preference to compound $B$, compound $B$ is more readily adsorbed on the column of $\mathrm{Al}_{2} \mathrm{O}_{3}$.
36: Why is sulfide ore of copper heated in a furnace after mixing with silica?
Ans: Sulfide ores are not reduced easily, but oxide ores are easily reduced. Sulfide ore of copper contains some types of impurities. This can cause by-product formation and degrade our final product. Iron oxide is present as a key impurity of concern in sulfide ore of copper. During the roasting process the temperature in the furnace is near about $1200^{\circ} \mathrm{C}$. During such high temperatures iron oxide which is gangue (impurity) in ore forms slag. Slag is insoluble in molten metal and being lighter floats over the surface of molten metal. Here silica is used as flux.
$\mathrm{FeO}+\mathrm{SiO}_{2} \rightarrow \mathrm{FeSiO}_{3}$
37. Why are sulfide ores converted to oxide before reduction?
Ans: Sulfide ore of copper contains some types of impurities. Sulfide ores are not reduced easily, but oxide ores are easily reduced. It is necessary to make sulfide ores free from volatile impurities. This can be performed by a roasting method. In this moisture escape and impurities like sulfur, phosphorus, arsenic are oxidized to their volatile oxides. It is performed in a reverberatory furnace. After this process the mass becomes porous.
38. Which method is used for refining Zr and Ti? Explain with equations.
Ans: Vapor phase refining or Van Arkel's method is used for refining Zr and Ti.
Vapor refining or vapor phase process: The metal is collected in the form of volatile compound that decomposes to pure metal.
Van Arkel method: This method is useful for removing all Oxygen and nitrogen present in the form of impurity in metals like $\mathrm{Zr}$ and Ti. The crude metal is heated in an empty vessel with a small amount of iodine. The metal iodine being more covalent volatilises. Impure $\mathrm{Zr}$ reacts with iodine to form zirconium tera iodide.
The following reaction occurs $\mathrm{Zr}+2 \mathrm{I}_{2} \rightarrow \mathrm{ZrI}_{4}$ The metal iodide is decomposed on a tungsten filament electrically heated to about $1800 \mathrm{~K}$. The pure metal is deposited on the filament and to maintain temperature the current is steadily raised, as the depositing continues.
39. What should be the considerations during the extraction of metals by electrochemical method?
Ans: The considerations during the extraction of metals by electrochemical method are :
a) Reactivity of metals: If the metals are reactive and are likely to react with water then the metals should be isolated by the electrolysis of their purified molten ore rather than their aqueous solution.
b) Suitability of electrodes: The electrode is carefully chosen so that they should not react with the product of electrolysis. If they react then the electrodes must be made of a material which is quite cheap so their replacement should not increase the cost of the process.
40. What is the role of flux in metallurgical processes?
Ans: In metallurgical processes, Flux works as various types of agents like chemical agents, cleaning/purifying agents.
Fluxes have many important properties such as corrosivity, cleanability, conductivity, volatility, etc.
Flux, when combined with gangue together, forms slag i.e. Flux + Gangue = Slag
As compared to gangue, slag separates much easily from the ore, in this way the removal of gangue also becomes much easier.
Flux is useful for making the molten mass more conducting which helps to remove impurities from metals through electrolytic metallurgical processes.
41. How are metals used as semiconductors refined? What is the principle of the method used?
Ans: Semiconducting metal is produced by the zone refining method.
Zone refining is centered on the principle that the impurities which are included in the metal ores are more soluble in the molten state as compared to the solid-state of metals.
Zone refining is mostly used for obtaining semiconductor and other metals which are highly pure, e.g., germanium, silicon, etc.
42. Write down the reactions taking place in Blast furnaces related to the metallurgy of iron in the temperature range 500-800 $\mathrm{K}$.
Ans: The chemical reactions taking place in Blast furnace in the temperature range 500-800 $\mathrm{K}$ are:
$3 \mathrm{Fe}_{2} \mathrm{O}_{3}+\mathrm{CO} \rightarrow 2 \mathrm{Fe}_{3} \mathrm{O}_{4}+\mathrm{CO}_{2}$
$\mathrm{Fe}_{3} \mathrm{O}_{4}+4 \mathrm{CO} \rightarrow 3 \mathrm{Fe}+4 \mathrm{CO}_{2}$
$\mathrm{Fe}_{2} \mathrm{O}_{3}+\mathrm{CO} \rightarrow 2 \mathrm{FeO}+\mathrm{CO}_{2}$
43. Give two requirements for vapor phase refining.
Ans: The two import requirements for performing the Vapor Phase Refining Method:
a) The metal which is to be refined should form a volatile compound with an available reagent.
b) The volatile compound formed must be easily decomposable so that it is easy to recover the desired metal.
44. Write the chemical reactions involved in the extraction of gold by cyanide process. Also give the role of zinc in the extraction.
Ans: The metal gold is a nonreactive metal. It is found as a native metal in its free state.
So, it is not compulsory to separate it by a chemical process. Thus, gold is leached to get it in its pure form. In the metallurgy of gold(Au), the respective metal is leached with a dilute mixture of NaCN or KCN in the presence of air where Gold(Au) is oxidized by oxygen of the air to $\mathrm{Au}^{+}$cation which then merges with ions to form a soluble compound.
$4 \mathrm{Au}(s)+8 \mathrm{CN}^{-}(a q)+\mathrm{O}_{2}^{-}(g)+2 \mathrm{H}_{2} \mathrm{O}^{-}(a q) \rightarrow 4\left[\mathrm{Au}(\mathrm{CN})_{2}\right]^{-}(a q)+4 \mathrm{OH}^{-}(a q)$
Gold is then obtained from this soluble compound $\mathrm{x}$ by replacement method by using a much more electropositive Zinc metal.
In this method of Gold extraction, the metal Zinc (Zn) plays the role of a reducing agent and it reduces $\mathrm{Au}^{+}$to $\mathrm{Au}$.
$\mathrm{Zn}$ itself gets oxidized to $\mathrm{Zn}^{+}$ions which combine with $\mathrm{CN}^{-}$ions to form another soluble complex. $2 \mathrm{Na}\left[\mathrm{Au}(\mathrm{CN})_{2}\right](\mathrm{aq})+\mathrm{Zn} \rightarrow 2 \mathrm{Au}+\mathrm{Na}_{2}\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]$.
Matching Type
45. Match the items of Column I with items of Column II and assign the correct code:
Column I | Column II |
(A) Pendulum | (1) Chrome steel |
(B) Malachite | (2) Nickel steel |
(C) Calamine | (3) $\mathrm{Na}_{3} \mathrm{AlF}_{6}$ |
(D) Cryolite | (4) $\mathrm{CuCO}_{3} \cdot \mathrm{Cu}(\mathrm{OH})_{2}$ |
(5) $\mathrm{ZnCO}_{3}$ |
Code:
(i) $A$ (1) $B$ (2) C (3) D (4)
(ii) $A$ (2) $B$ (4) C (5) D (3)
(iii) $A$ (2) $B$ (3) $C$ (4) $D$ (5)
(iv) $A$ (4) $B$ (5) C (3) D (2)
Ans: Option (ii) A (2) B (4) C (5) D (3)
Pendulum is always made of nickel steel.
Hence, option (A) from column I is matched with option (2) from column II. Malachite is the ore of copper.
Hence, option (B) from column I is matched with option (4) from column II. Calamine is the ore of zinc.
Hence, option (C) from column I is matched with option (5) from column II. Cryolite is an ore of aluminum.
Hence, option (D) from column I is matched with option (3) from column II.
46. Match the items of Column I with the items of Column II and assign the correct code:
Column I | Column II |
(A) Coloured bands | (1) Zone refining |
(B) Impure metal to volatile complex | (2) Fractional distillation |
(C) Purification of Ge and Si | (3) Mond process |
(D) Purification of mercury | (4) Chromatography |
(5) Liquation |
(i) $\quad A(1) B(2) C(4) D(5)$
(ii) $\quad A(4) B(3) C(1) D(2)$
(iii) $\quad A(3) B(4) C(2) D(1)$
(iv) $\quad A(5) B(4) C(3) D(2)$
Ans: Option (ii) A (4) B (3) C (1) D (2)
Coloured bands are found in chromatography.
Hence, option (A) from column I is matched with option (4) from column II. Impure metals are converted to volatile complexes in Mond's process.
Hence, option (B) from column I is matched with option (3) from column II. Purification of Ge and silicon is done using zone refining.
Hence, option (C) from column I is matched with option (1) from column II. Purification of mercury is done using fractional distillation.
Hence, option (D) from column I is matched with option (2) from column II.
47. Match items of Column I with the items of Column II and assign the correct code :
Column I | Column II |
(A) Cyanide process | (1) Ultrapure Ge |
(B) Froth Floatation Process | (2) Dressing of ZnS |
(C) Electrolytic reduction | (3) Extraction of Al |
(D) Zone refining | (4) Extraction of Au |
(5) Purification of Ni |
(i) A(4) B(2) C(3) D(1)
(ii) A(2) B(3) C(1) D(5)
(iii) A(1) B(2) C(3) D(4)
(iv) A(3) B(4) C(5) D(1)
Ans: Correct code (i) A (4) B (2) C (3) D (1)
The cyanide process is used in the extraction of Au.
Hence, option (A) from column I is matched with option (4) from column II. Froth floatation process is used in the dressing of $\mathrm{ZnS}$.
Hence, option (B) from column I is matched with option (2) from column II. Electrolytic reduction is used in the extraction of AI.
Hence, option (C) from column I is matched with option (3) from column II. Zone refining is used to get ultrapure Ge.
Hence, option (D) from column I is matched with option (1) from column II.
48. Match the items of Column I with the items of Column II and assign the correct code :
Column I | Column II |
(A) Sapphire | (1) $\mathrm{Al}_{2} \mathrm{O}_{3}$ |
(B) Sphalerite | (2) $\mathrm{NaCN}$ |
(C) Depressant | (3) Co |
(D) Corundum | (4) ZnS |
(5) $\mathrm{Fe}_{2} \mathrm{O}_{3}$ |
(i) $A(3) B(4) C(2) D(1)$
(ii) $A(5) B(4) C(3) D(2)$
(iii) $A(2) B(3) C(4) D(5)$
(iv) $A(1) B(2) C(3) D(4)$
Ans: Option (i) A (3) B (4) C (2) D (1)
Sapphire is a gemstone containing Co.
Hence, option (A) from column I is matched with option (3) from column II. The Sphalerite single is $\mathrm{ZnS}$.
Hence, option (B) from column I is matched with option (4) from column II. NaCN is also used as a depressant.
Hence, option (C) from column I is matched with option (2) from column II. $\mathrm{Al}_{2} \mathrm{O}_{3}$ is also called corundum.
Hence, option (D) from column I is matched with option (1) from column II.
49: Match the items of Column I with items of Column II and assign the correct code :
Column I | Column II |
(A) Blistered Cu | (1) Aluminum |
(B) Blast furnace | (2) $2 \mathrm{Cu}_{2} \mathrm{O}+\mathrm{Cu}_{2} \mathrm{~S} \rightarrow 6 \mathrm{Cu}+\mathrm{SO}_{2}$ |
(C) Reverberatory furnace | (3) Iron |
(D) Hall-Heroult process | (4) $\mathrm{FeO}+\mathrm{SiO}_{2} \rightarrow \mathrm{FeSiO}_{3}$ |
(5) $2 \mathrm{Cu}_{2} \mathrm{~S}+3 \mathrm{O}_{2} \rightarrow 2 \mathrm{Cu}_{2} \mathrm{O}+2 \mathrm{SO}_{2}$ |
Code:
(i) $\quad A$ (2) $B$ (3) C (4) D (1)
(ii) $\quad A$ (1) $B$ (2) C (3) D (5)
(iii) $\quad A(5) B(4) C(3) D(2)$
(iv) $A(4) B(5) C(3) D(2)$
Ans: Option (i) A (2) B (3) C (4) D (1)
Explanation: A solidified copper has a blistered appearance due to the evolution of $\mathrm{SO}_{2}$ Hence it is called blistered copper.
Hence, option (A) from column I is matched with option (2) from column II. Iron is extracted from a blast furnace.
Hence, option (B) from column I is matched with option (3) from column II. The iron ore is heated in the reverberatory furnace after mixing with silica. In the furnace, iron oxide slags of iron and copper are produced in the form of copper matte.
Hence, option (C) from column I is matched with option (4) from column II. The hall-Heroult process is used for the extraction of aluminum from its ore.
Hence, option (D) from column I is matched with option (1) from column II.
Assertion and Reason Type
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Both assertion and reason are true and reason is the correct explanation of assertion.
(ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
(iii) Assertion is true but the reason is false.
(iv) Assertion is false but the reason is true.
(v) Assertion and reason both are wrong.
50. Assertion: Nickel can be purified by the Mond process.
Reason: $\mathrm{Ni}(\mathrm{CO})_{4}$ is a volatile compound which decomposes at $460 \mathrm{~K}$ to give pure $\mathrm{Ni}$.
Ans: (i) Both assertion and reason are true and reason is the correct explanation of assertion.
Explanation: In the Mond process Nickel is reacted with carbon monoxide reversibly to give Nickel carbonyl, $\mathrm{Ni}(\mathrm{CO})_{4}$. Nickel carbonyl is a volatile compound. It decomposes to nickel and carbon monoxide at $460 \mathrm{~K}$.
51. Assertion: Zirconium can be purified by Van Arkel method.
Reason: $\mathrm{ZrI}_{4}$ is volatile and decomposes at $1800 \mathrm{~K}$.
Ans: (i) Both assertion and reason are true and reason is the correct explanation of assertion.
Explanation: Van Arkel method is generally used to obtain pure forms of Zirconium (Zr) and Titanium (Ti).
The metal iodide is heated at $1800 \mathrm{~K}$ and is decomposed on a tungsten filament. The pure metal is dropped on the tungsten filament. This proves that $\mathrm{ZrI}_{4}$ is volatile and decomposes at $1800 \mathrm{~K}$.
52. Assertion: Sulfide ores are concentrated by Froth Flotation method.
Reason: Cresols stabilize the froth in the Froth Flotation method.
Ans: (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: Froth Flotation method is used to separate the hydrophobic materials from hydrophilic materials. In this method a mixture of palm oil, water and detergent is taken in a tank along with powdered sulfide ore. Compressed air is then passed through the pipe of the rotating agitator to create froth. The sulfide ore is then wetted by the palm oil mixture and it rises with the froth and the impurities or gauge settles at the bottom of the tank. The froth containing the sulfide is then cleaned and dried. Cresols or Aniline are used to stabilize the froth and in case of sulfides palm oil is used to collect non wet particles of the sulfide.
53. Assertion: Zone refining method is very useful for producing semiconductors.
Reason: Semiconductors are of high purity.
Ans: (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: The zone refining method is very useful for producing high-purity semiconductors and other metals, such as germanium.
54. Assertion: Hydrometallurgy involves dissolving the ore in a suitable reagent followed by precipitation by a more electropositive metal.
Reason: Copper is extracted by hydrometallurgy.
Ans: (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: Hydrometallurgy is used to extract copper from low-grade ore. Hydrometallurgy entails dissolving the ore in a suitable reagent and then precipitating it. In this method, more electropositive metal is used, allowing pure metal to be displaced.
Long Answer Type
55. Explain the following :
(a) $\mathrm{CO}_{2}$ is a better reducing agent below $710 \mathrm{~K}$ whereas $\mathrm{CO}$ is a better reducing agent above $710 \mathrm{~K}$.
(b) Generally, sulfide ores are converted into oxides before reduction.
(c) Silica is added to the sulfide ore of copper in the reverberatory furnace.
(d) Carbon and hydrogen are not used as reducing agents at high temperatures.
(e) Vapor phase refining method is used for the purification of Ti.
Ans:
(a). According to Ellingham diagram, the reaction of $\mathrm{CO}_{2}$ is more feasible at temperatures lower than $710 \mathrm{~K}$ and thus it is a better reducing agent below $710 \mathrm{~K}$.
While the reaction of $\mathrm{CO}$ is more feasible at temperatures higher than $710 \mathrm{~K}$ and thus it is a better reducing agent at above $710 \mathrm{~K}$.
(b). According to the Ellingham diagram, the more negative the Gibbs free energy of a particular reaction the more feasible it is to carry out. Since the oxides are easier to reduce, sulfide ores are converted into oxides before reduction.
(c). To extract copper, its sulfide ores are supposed to be heated in a reverberatory furnace. The sulfide ore may contain iron as an impurity. So, it is mixed with silica before heating. The oxide of iron slags off as iron silicate. In other words, the iron impurity is removed easily in the form of iron silicate.
(d). Carbon and hydrogen are good reducing agents. They are used preferably for reduction of oxides. They also form escapable gasses as by-products. However, at higher temperatures they form carbides and hydrides. Also, it is not convenient to maintain such high temperatures. Thus, electrolysis is used for this purpose.
(e). Ti is heated in an evacuated vessel along with iodine. Ti forms volatile compounds with iodine. The volatile compound formed decomposes easily. This makes the recovery of the metal convenient. Ti thus fulfills the requirements needed for carrying out vapor phase refining. Hence, this method is used for its purification.
General Principles and Processes of Isolation of Elements is a very scoring Chapter. It has some of the easiest equations and reactions that can be memorized by students of Class 12 very easily by simply reviewing the content of the topic every once in a while. In order to score the most in this Chapter, students are advised to be well aware of all the necessary steps that are involved in the extraction and refining of several elements. Moreover, students of Class 12 are also advised to make sure that they know the chemical names and different terminologies used in the Chapter before taking the final test.
Benefits of Using NCERT Exemplar book solutions for Chapter 6 General Principles and Processes of Isolation of Elements
Vedantu has combined the best answers possible in order to bring this free PDF solutions for students of Class 12 for the Chapter General Principles and Processes of Isolation of Elements. By using this free resource, students can:-
1) Get an idea of questions asked in School-level Exams and competitive Exams from the Chapter General Principles and Processes of Isolation of Elements
2) Understand the way in which answers are written in the final test
3) Be confident as the NCERT Exemplar has shown many trends where questions appear directly from it in several competitive and school-level Exams
4) Manage their time well and score more on their mock tests
FAQs on NCERT Exemplar for Class 12 Chemistry Chapter-6 (Book Solutions)
1. Should I study from NCERT Exemplar for Class 12 Chapter 6 General Principles and Processes of Isolation of Elements?
NCERT Exemplar, as already mentioned, has proved to be accurate and has given several good quality questions to students of Class 12. These questions have appeared directly in many competitive Exams and even school-level Examinations too. By studying from the NCERT Exemplar, students can get a good idea of the types of questions asked in the final test and then have a higher level of confidence when it comes to the final test. They can score high marks in the Chapter by using the NCERT Exemplar Solutions for Chapter 6.
2. How do I memorize important reactions from Class 12 Chapter 6 General Principles and Processes of Isolation of Elements?
Memorizing equations and reactions from Class 12 Chapter 6 General Principles and Processes of Isolation of Elements is not very difficult. One easy way in which students can approach this is by making a list of all the equations and reactions that are involved in several processes that happen in the Chapter General Principles and Processes of Isolation of Elements. By reviewing this list of reactions on a daily basis, students can memorize the reactions involved with ease and can be more confident with their ability to score more in the Chapter General Principles and Processes of Isolation of Elements.
3. How do I revise the entire Chapter of General Principles and Processes of Isolation of Elements for Class 12 in one go?
To finish the revision of an entire Chapter in one go, students must know what resource they must be referring to. It is a necessity to make sure that students understand the basic concept with ease and not get bored at the same time. Luckily, Vedantu offers free resources to students to make sure that their preparation does not stop.
Here is a video that covers the entire Chapter of General Principles and Processes of Isolation of Elements from Vedantu.
4. What are some of the most important topics that need to be covered from the Class 12 Chapter 6 General Principles and Processes of Isolation of Elements?
The most important topics that need to be covered from the Chapter General Principles and Processes of Isolation of Elements are:-
Pulverization and concentration of ore
Extraction of crude metal from concentrated ore
Refining of crude metals
Occurrence and Principle of extraction of aluminium from bauxite
Occurrence and Principle of extraction of copper from copper pyrites
Occurrence and Principle of extraction of iron from haematite
It is recommended to study these topics well and make sure that students are also learning all the important equations and reactions involved.
5. How much time would it take to cover Class 12 Chapter 6 General Principles and Processes of Isolation of Elements from the NCERT Exemplar?
The time invested in a Chapter is going to vary from one student to another. However, one thing that is common among all students of Class 12 when it comes to studying General Principles and Processes of Isolation of Elements is that it is considered one of the easiest Chapters in the course. Which is very true. The time that is invested in this Chapter may be less compared to other Chapters, but students will surely get great marks!

























