
Two air bubbles of radii \[2\]\[mm\]and \[4\]\[mm\], formed in the same liquid come together to form a big bubble. If the surface tension of the liquid is \[0.70\] \[N{m^{ - 1}}\], the radius of curvature of common interface to both bubbles will be:
A. \[6\]\[mm\] with concave surface towards smaller bubble
B. \[2\]\[mm\] with concave surface towards bigger bubble
C. \[4\]\[mm\] with concave surface towards smaller bubble
D. \[4\]\[mm\] with concave surface towards bigger bubble
Answer
580.2k+ views
Hint: Formation of air bubble: The surface tension of water provides the necessary wall tension for the formation of bubbles with water. The tendency to minimize that wall tension pulls the bubbles into spherical shapes.
The pressure difference between the inside and outside of a bubble depends upon the surface tension and the radius of the bubble. The relationship can be obtained by visualizing the bubble as two hemispheres and noting that the internal pressure which tends to push the hemispheres apart is connected by the surface tension acting around the circumference of the circle.
For a bubble with two surfaces providing tension, the pressure relationship is: \[{P_i} - {P_0} = \dfrac{{4T}}{r}\]
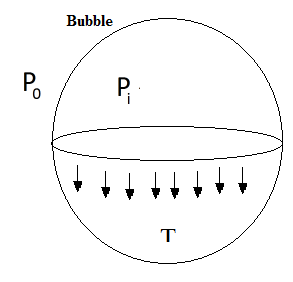
Complete step by step answer:
If \[{P_0}\]is the atmospheric pressure, then \[{P_i}\]inside the smaller bubble and \[{P_2}\]inside the bigger bubble are given by
\[{P_i} - {P_0} = \dfrac{{4T}}{{{r_1}}}\] and \[{P_2} - {P_0} = \dfrac{{4T}}{{{r_2}}}\]
The pressure is more inside the smaller bubble. When, double bubble is formed, the interface will be concave towards the smaller bubble side.
The exceed pressure corresponding this surface is given as
\[P = {P_i} - {P_2} = \dfrac{{4T}}{R}\]
Here, = radius of curvature of the internal film. On substituting
\[\left( {{P_0} + \dfrac{{4T}}{{{r_1}}}} \right) - \left( {{P_0} + \dfrac{{4T}}{{{r_2}}}} \right) = \dfrac{{4T}}{R}\]
or \[\dfrac{1}{{{r_1}}} - \dfrac{1}{{{r_2}}} = \dfrac{1}{R}\]
\[R = \dfrac{{{r_1}{r_2}}}{{{r_2} - {r_1}}}\]
$\therefore R = \dfrac{{2 \times 4}}{{4 - 2}} = 4$ \[mm\]
We know that the excess pressure is more towards the concave surface and is inversely proportional to radius of the bubble, therefore the concave surface bubble as shown by dotted curve.
So, the correct answer is “Option C”.
Additional Information:
Surface tension exists because at the surface of the liquid, solid, or gas there is a difference in pressure from the rest of the material. In the volume of the material, there is more pressure from the surroundings, pressing the molecules closer to each other, so that the force they put on each other is slightly repulsive. However, on the surface of the material, there is much less pressure, and so the atoms are farther apart, creating a more attractive interaction. This stronger attraction is what gives rise to surface tension.
Note:
Students make mistakes by choosing the direction of excess pressure. As the excess pressure is always towards the concave surface and pressure in the smaller bubble is greater than that in the larger bubble, the common surface is concave towards the center of the smaller bubble.
The pressure difference between the inside and outside of a bubble depends upon the surface tension and the radius of the bubble. The relationship can be obtained by visualizing the bubble as two hemispheres and noting that the internal pressure which tends to push the hemispheres apart is connected by the surface tension acting around the circumference of the circle.
For a bubble with two surfaces providing tension, the pressure relationship is: \[{P_i} - {P_0} = \dfrac{{4T}}{r}\]

Complete step by step answer:
If \[{P_0}\]is the atmospheric pressure, then \[{P_i}\]inside the smaller bubble and \[{P_2}\]inside the bigger bubble are given by
\[{P_i} - {P_0} = \dfrac{{4T}}{{{r_1}}}\] and \[{P_2} - {P_0} = \dfrac{{4T}}{{{r_2}}}\]
The pressure is more inside the smaller bubble. When, double bubble is formed, the interface will be concave towards the smaller bubble side.
The exceed pressure corresponding this surface is given as
\[P = {P_i} - {P_2} = \dfrac{{4T}}{R}\]
Here, = radius of curvature of the internal film. On substituting
\[\left( {{P_0} + \dfrac{{4T}}{{{r_1}}}} \right) - \left( {{P_0} + \dfrac{{4T}}{{{r_2}}}} \right) = \dfrac{{4T}}{R}\]
or \[\dfrac{1}{{{r_1}}} - \dfrac{1}{{{r_2}}} = \dfrac{1}{R}\]
\[R = \dfrac{{{r_1}{r_2}}}{{{r_2} - {r_1}}}\]
$\therefore R = \dfrac{{2 \times 4}}{{4 - 2}} = 4$ \[mm\]
We know that the excess pressure is more towards the concave surface and is inversely proportional to radius of the bubble, therefore the concave surface bubble as shown by dotted curve.
So, the correct answer is “Option C”.
Additional Information:
Surface tension exists because at the surface of the liquid, solid, or gas there is a difference in pressure from the rest of the material. In the volume of the material, there is more pressure from the surroundings, pressing the molecules closer to each other, so that the force they put on each other is slightly repulsive. However, on the surface of the material, there is much less pressure, and so the atoms are farther apart, creating a more attractive interaction. This stronger attraction is what gives rise to surface tension.
Note:
Students make mistakes by choosing the direction of excess pressure. As the excess pressure is always towards the concave surface and pressure in the smaller bubble is greater than that in the larger bubble, the common surface is concave towards the center of the smaller bubble.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




