
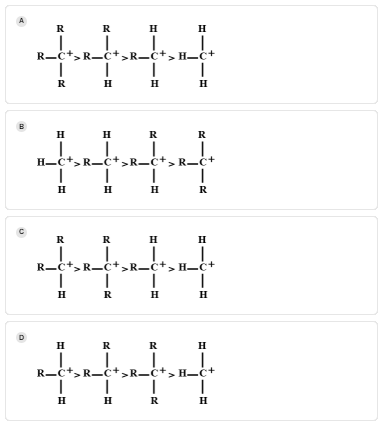
The decreasing order of stability of alkyl carbocation is:
Answer
233.1k+ views
Hint: As we know that carbocation is a group of atoms in which a carbon atom is positively charged. Carbocation is an electron deficient species.
Complete answer:
As tertiary carbocation contains three alkyl groups, so they have three methyl groups to distribute its positive charge than primary and secondary carbocation because they both contain one and two alkyl groups respectively. Three H-atoms are attached in the methyl group but adjacent hydrogen does not stabilize carbocation. So, the decreasing order of stability of carbocation is as follows:
Tertiary > secondary > primary > methyl
Hence, the correct answer is A.
Additional Information:
In carbocation, the hybridization of carbon is and its shape is trigonal planar. The empty p orbital indicates that it has an electron-deficient nature. The carbon has 6 electrons in its valence shell. Carbocation is formed by two ways they are cleavage of bonds of carbon and electrophilic addition. The cleavage of the carbon bond and the atoms attached to it takes away the shared electrons from the leaving group. And it makes the carbon atom deficient as an electron. As a result, a positive charge is formed which is known as carbocation. An electrophile attacks on an unsaturated point (that is double or triple bond) in electrophilic addition, this results in the cleavage of the pi bond which results in the formation of a carbocation.
Note:
Students might confuse among primary, secondary and tertiary alkyl groups. Primary carbon is bonded to one other carbon while tertiary and secondary are bonded to three and two carbon respectively.
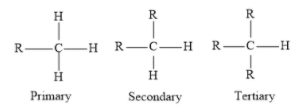
Complete answer:
As tertiary carbocation contains three alkyl groups, so they have three methyl groups to distribute its positive charge than primary and secondary carbocation because they both contain one and two alkyl groups respectively. Three H-atoms are attached in the methyl group but adjacent hydrogen does not stabilize carbocation. So, the decreasing order of stability of carbocation is as follows:
Tertiary > secondary > primary > methyl

Hence, the correct answer is A.
Additional Information:
In carbocation, the hybridization of carbon is and its shape is trigonal planar. The empty p orbital indicates that it has an electron-deficient nature. The carbon has 6 electrons in its valence shell. Carbocation is formed by two ways they are cleavage of bonds of carbon and electrophilic addition. The cleavage of the carbon bond and the atoms attached to it takes away the shared electrons from the leaving group. And it makes the carbon atom deficient as an electron. As a result, a positive charge is formed which is known as carbocation. An electrophile attacks on an unsaturated point (that is double or triple bond) in electrophilic addition, this results in the cleavage of the pi bond which results in the formation of a carbocation.
Note:
Students might confuse among primary, secondary and tertiary alkyl groups. Primary carbon is bonded to one other carbon while tertiary and secondary are bonded to three and two carbon respectively.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




