
The correct order increasing acidic strength is:
(A) Phenol (B) ethanol< Phenol< chloroacetic acid (C) ethanol (D) chloroacetic acid< acetic acid
Answer
524.8k+ views
Hint: Let’s see the definition of acid strength- Acid strength is the tendency of an acid HA , to dissociate into a proton${{H}^{+}}$, and an anion, ${{A}^{-}}$. Acid strength depends on many factors like inductive effect, resonance effect, electronegativity of atoms etc.
Step by step solution:
- The acidic character will be least in ethanol because, here the $C{{H}_{3}}C{{H}_{2}}$$C{{H}_{3}}C{{H}_{2}}$group is present, which is a +I group and are electron donating groups which decreases the separation of OH bond and hence is least acidic.
\[C{{H}_{3}}C{{H}_{2}}OH\]
- Now, next will be phenol which is slightly more acidic than ethanol. Because in phenol, the ${{C}_{6}}{{H}_{5}}$group present here is a – I group that is an electron withdrawing group, that will increase the polarity of the OH bond or we can say increase the separation of OH bond. And hence is more acidic than ethanol.
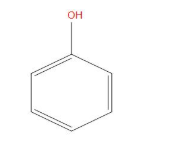
- Now, next will be acetic acid, which is more acidic than phenol. This is because when the acidic hydrogen is removed then the negative charge formed on oxygen basically show resonance with the adjoining oxygen atom, and hence by showing resonance it will become stable and more acidic than that of phenol.
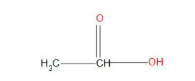
- Among all the given compounds chloroacetic acid will be more acidic, this is because this can show resonance as well as chlorine is present which will show –I effect , in this effect the electron withdrawing group that is Cl will start withdrawing electrons from the adjacent carbon atom , and hence this will assist dissociation of the OH bond and more easily the hydrogen will get separated . And hence, will show the highest acidic strength than all other.
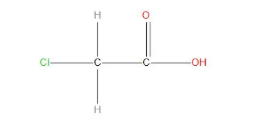
- We can see that the resonating structure of acetic acid is:
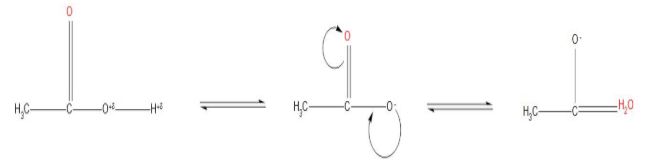
Hence, we can conclude that the correct option will be(c) that is ethanol
Additional information:
There are various factors affecting acid strength. Some of them are:
- It depends on the strength of the H-A bond. The weaker the bond, the lesser the energy will be required to break the bond. Hence, the acid is strong. It is also found to be dependent on inductive effect.
- Next factor is the polarity of the H-A bond, it also affects its acid strength. If the bond is highly polar, then it is seen that the proton tends to leave the molecule more easily, making it a strong acid.
- Inductive effect shows major role in deciding the acidic nature. +I effect and electron donating groups will decrease the acidic nature. -I effect and electron withdrawing group will increase the acidic nature.
Note:
- The acidic strength of various functional groups are found in order of: Carboxylic Acid and Acid Anhydride > Phenol > Alcohol >>> Amine.
- While checking the acidic strength, one should check all the factors that are affecting acid strength.
Step by step solution:
- The acidic character will be least in ethanol because, here the $C{{H}_{3}}C{{H}_{2}}$$C{{H}_{3}}C{{H}_{2}}$group is present, which is a +I group and are electron donating groups which decreases the separation of OH bond and hence is least acidic.
\[C{{H}_{3}}C{{H}_{2}}OH\]
- Now, next will be phenol which is slightly more acidic than ethanol. Because in phenol, the ${{C}_{6}}{{H}_{5}}$group present here is a – I group that is an electron withdrawing group, that will increase the polarity of the OH bond or we can say increase the separation of OH bond. And hence is more acidic than ethanol.

- Now, next will be acetic acid, which is more acidic than phenol. This is because when the acidic hydrogen is removed then the negative charge formed on oxygen basically show resonance with the adjoining oxygen atom, and hence by showing resonance it will become stable and more acidic than that of phenol.

- Among all the given compounds chloroacetic acid will be more acidic, this is because this can show resonance as well as chlorine is present which will show –I effect , in this effect the electron withdrawing group that is Cl will start withdrawing electrons from the adjacent carbon atom , and hence this will assist dissociation of the OH bond and more easily the hydrogen will get separated . And hence, will show the highest acidic strength than all other.

- We can see that the resonating structure of acetic acid is:

Hence, we can conclude that the correct option will be(c) that is ethanol
Additional information:
There are various factors affecting acid strength. Some of them are:
- It depends on the strength of the H-A bond. The weaker the bond, the lesser the energy will be required to break the bond. Hence, the acid is strong. It is also found to be dependent on inductive effect.
- Next factor is the polarity of the H-A bond, it also affects its acid strength. If the bond is highly polar, then it is seen that the proton tends to leave the molecule more easily, making it a strong acid.
- Inductive effect shows major role in deciding the acidic nature. +I effect and electron donating groups will decrease the acidic nature. -I effect and electron withdrawing group will increase the acidic nature.
Note:
- The acidic strength of various functional groups are found in order of: Carboxylic Acid and Acid Anhydride > Phenol > Alcohol >>> Amine.
- While checking the acidic strength, one should check all the factors that are affecting acid strength.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




