
When sulphur monochloride is saturated with chlorine, the compound formed is
A) $SC{{l}_{6}}$
B) $SC{{l}_{4}}$
C) $SC{{l}_{2}}$
D) ${{S}_{2}}C{{l}_{2}}$
Answer
233.1k+ views
Hint: Sulphur has 2 free electrons in the outermost subshell to get covalent bonding in ground state and in an excited state it has 6 electrons free in its last shell. So, in less or saturated amount of chlorine sulphur be in ground state.
Complete step by step solution:
Now formula of sulphur monochloride is ${{S}_{2}}C{{l}_{2}}$
And electronic configuration of $S$in ground state be like that shown in diagram
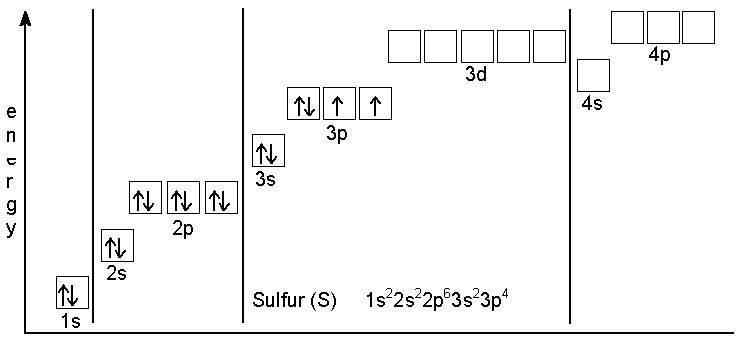
There are two atoms in last $3p$ subshell when sulphur is in ground state so sulphur breaks from its $S-S$ Bond in ${{S}_{2}}C{{l}_{2}}$ and make a coordinate bond with $Cl$ in which there is a last vacant shell in outer subshell.
Electronic configuration of chlorine is \[\left[ Ne \right]\text{ }3s{}^\text{2}\text{ }3{{p}^{5}}\]
So, the reaction be like-
\[{{S}_{2}}C{{l}_{2}}+C{{l}_{2}}\to SC{{l}_{2}}\]
Then in the excess or in saturated quantity of chlorine gas it gives sulphur dioxide as a product.
Hence Option (C) is correct.
Additional Information:
Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula ${{S}_{2}}C{{l}_{2}}$ Some alternative names for this compound are sulfur monochloride, disulphur dichloride and sulphur monochloride.Its structure be like:
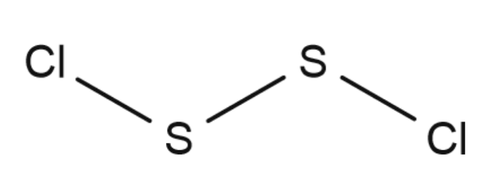
There is a covalent bond between sulphur and sulphur to complete inertness of its outer shell.
It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:
\[{{S}_{8}}~+\text{ }4\text{ }C{{l}_{2}}~\to \text{ }4\text{ }{{S}_{2}}C{{l}_{2}},~\Delta H~=\text{ }-58.2~kJ/mol\]
Note: It gives different reactions when chlorine is not present in sufficient amounts. So the saturated word in question decides many factors in which direction the reaction goes. It also depends on the stability of the final product. Also, temperature during reaction also plays a major factor on the final product.
Complete step by step solution:
Now formula of sulphur monochloride is ${{S}_{2}}C{{l}_{2}}$
And electronic configuration of $S$in ground state be like that shown in diagram

There are two atoms in last $3p$ subshell when sulphur is in ground state so sulphur breaks from its $S-S$ Bond in ${{S}_{2}}C{{l}_{2}}$ and make a coordinate bond with $Cl$ in which there is a last vacant shell in outer subshell.
Electronic configuration of chlorine is \[\left[ Ne \right]\text{ }3s{}^\text{2}\text{ }3{{p}^{5}}\]
So, the reaction be like-
\[{{S}_{2}}C{{l}_{2}}+C{{l}_{2}}\to SC{{l}_{2}}\]
Then in the excess or in saturated quantity of chlorine gas it gives sulphur dioxide as a product.
Hence Option (C) is correct.
Additional Information:
Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula ${{S}_{2}}C{{l}_{2}}$ Some alternative names for this compound are sulfur monochloride, disulphur dichloride and sulphur monochloride.Its structure be like:

There is a covalent bond between sulphur and sulphur to complete inertness of its outer shell.
It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:
\[{{S}_{8}}~+\text{ }4\text{ }C{{l}_{2}}~\to \text{ }4\text{ }{{S}_{2}}C{{l}_{2}},~\Delta H~=\text{ }-58.2~kJ/mol\]
Note: It gives different reactions when chlorine is not present in sufficient amounts. So the saturated word in question decides many factors in which direction the reaction goes. It also depends on the stability of the final product. Also, temperature during reaction also plays a major factor on the final product.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
Understanding Average and RMS Value in Electrical Circuits

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Understanding Elastic Collisions in Two Dimensions

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

JEE Main Syllabus 2026: Download Detailed Subject-wise PDF

Other Pages
Alcohol Phenol and Ether Class 12 Chemistry Chapter 7 CBSE Notes - 2025-26

Haloalkanes and Haloarenes Class 12 Chemistry Chapter 6 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Understanding Collisions: Types and Examples for Students




