
How many \[\sigma \] and \[\pi \] bonds are there in the compound:
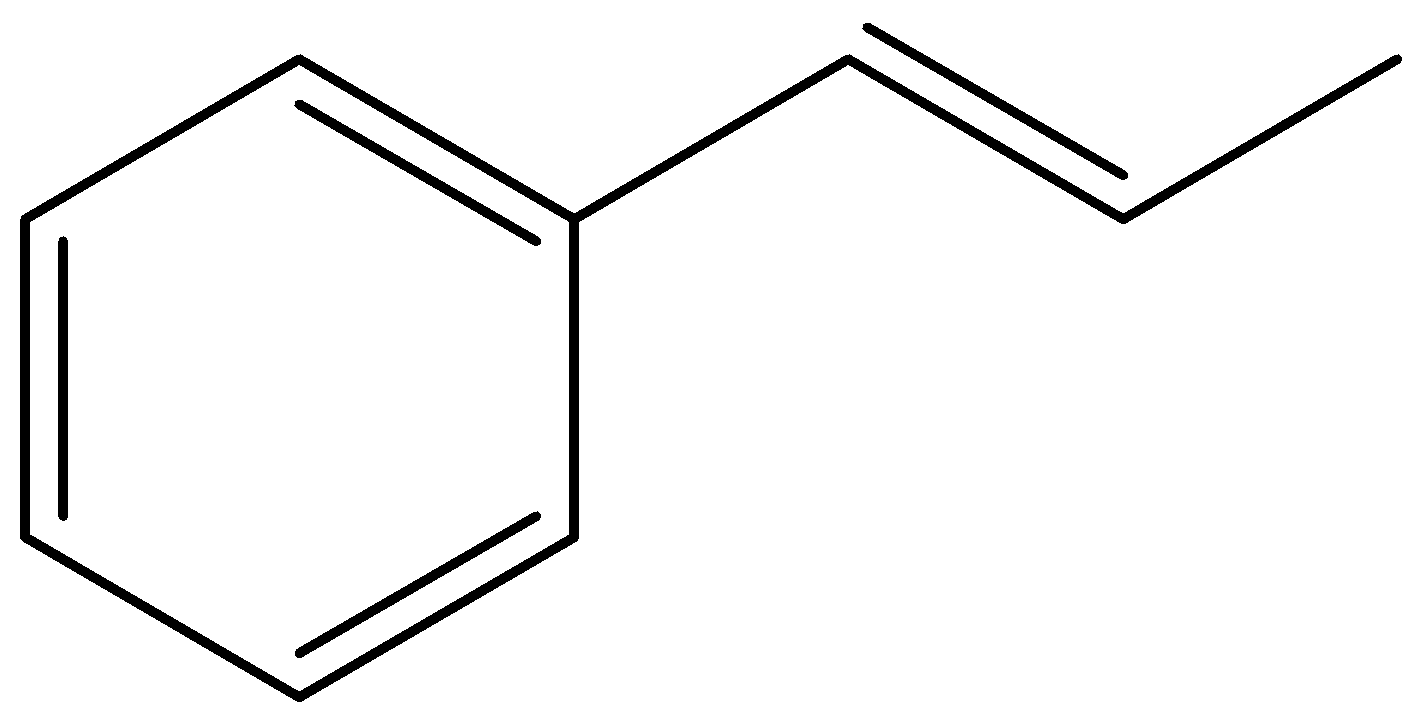
(A) 14\[\sigma \], 8\[\pi \]
(B) 18\[\sigma \],8\[\pi \]
(C) 19\[\sigma \],4\[\pi \]
(D) 14\[\sigma \],2\[\pi \]
Answer
233.1k+ views
Hint: If there is a single bond between any atom, then that bond can be considered as a \[\sigma \] bond. The multiplicity of a bond shows presence of a \[\pi \]-bond in addition to a \[\sigma \] bond.
Complete step by step solution:
A \[\pi \] bond is formed by overlap of orbitals in a side-by-side fashion and has zero electron density at its nodal planes. In other ways we can say that in a double bond, there is one \[\pi \]-bond and one \[\sigma \]-bond is present.
Let’s calculate the number of \[\pi \]-bonds present in the given compound.
You can see that there are 4 double bonds present in the compound and hence the number of \[\pi \]-bonds present in the given compound will be equal to 4.
Let’s find the number of \[\sigma \]-bonds present in the compound.
We can see that there are a total 19 \[\sigma \]-bonds present in the compound. Also draw C-H bonds and consider them in the counting of bonds as well.
So, correct answer is (C) 19\[\sigma \],4\[\pi \]
Additional Information:
A double bond is made up of one \[\sigma \]-bond and one \[\pi \]-bond. While a triple bond can be said to be made up of one \[\sigma \]-bond and two \[\pi \]-bonds.
\[\sigma \]-bond is more stronger than a \[\pi \]-bond.
Note:
Do not forget to include C-H \[\sigma \]-bonds in the counting process of total \[\sigma \]-bonds as they are often not shown in the structure and may lead to mistakes. Do not forget to include a \[\sigma \]-bond from a C-C double bond.
Complete step by step solution:
A \[\pi \] bond is formed by overlap of orbitals in a side-by-side fashion and has zero electron density at its nodal planes. In other ways we can say that in a double bond, there is one \[\pi \]-bond and one \[\sigma \]-bond is present.
Let’s calculate the number of \[\pi \]-bonds present in the given compound.
You can see that there are 4 double bonds present in the compound and hence the number of \[\pi \]-bonds present in the given compound will be equal to 4.
Let’s find the number of \[\sigma \]-bonds present in the compound.
We can see that there are a total 19 \[\sigma \]-bonds present in the compound. Also draw C-H bonds and consider them in the counting of bonds as well.
So, correct answer is (C) 19\[\sigma \],4\[\pi \]
Additional Information:
A double bond is made up of one \[\sigma \]-bond and one \[\pi \]-bond. While a triple bond can be said to be made up of one \[\sigma \]-bond and two \[\pi \]-bonds.
\[\sigma \]-bond is more stronger than a \[\pi \]-bond.
Note:
Do not forget to include C-H \[\sigma \]-bonds in the counting process of total \[\sigma \]-bonds as they are often not shown in the structure and may lead to mistakes. Do not forget to include a \[\sigma \]-bond from a C-C double bond.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




