
Propylene on hydrolysis with sulphuric acid forms
A. n-propyl alcohol
B. Isopropyl alcohol
C. Ethyl alcohol
D. Butyl alcohol
Answer
233.1k+ views
Hint: Alkenes react with dilute sulphuric acid to give alcohol. It is a hydration process where the addition of water to the alkene molecule takes place and the product formed will be alcohol. But pure water does not ionise so alkene does not react with it, thus some amount of acid is added to the water.
Complete Step by Step Answer:
On hydrolysis of alkene, the water molecule gets added to the alkene, and alcohol is formed. Since it is an addition reaction, this reaction will follow Markovnikov's law.
According to the addition mechanism of the alkene as the formation of carbocation takes place first the electrophile gets attached to the carbon which contains more no. of hydrogen atoms attached to the carbon and then the nucleophile gets attached to the carbon that contains less number of hydrogen atoms because according to markovnikov’s rule the nucleophile will attach with the carbon which contains less number of hydrogen.
In the addition reaction of alkene, the pi(π) bond between the two carbons of alkene breaks and they form two sigma bonds with two separate chemical species.
$CH_3-CH=CH_2 +\ \ dil.\ H_2SO_4\ \ \ \rightarrow CH_3-CH(OH)-CH_3$
Mechanism of the reaction:
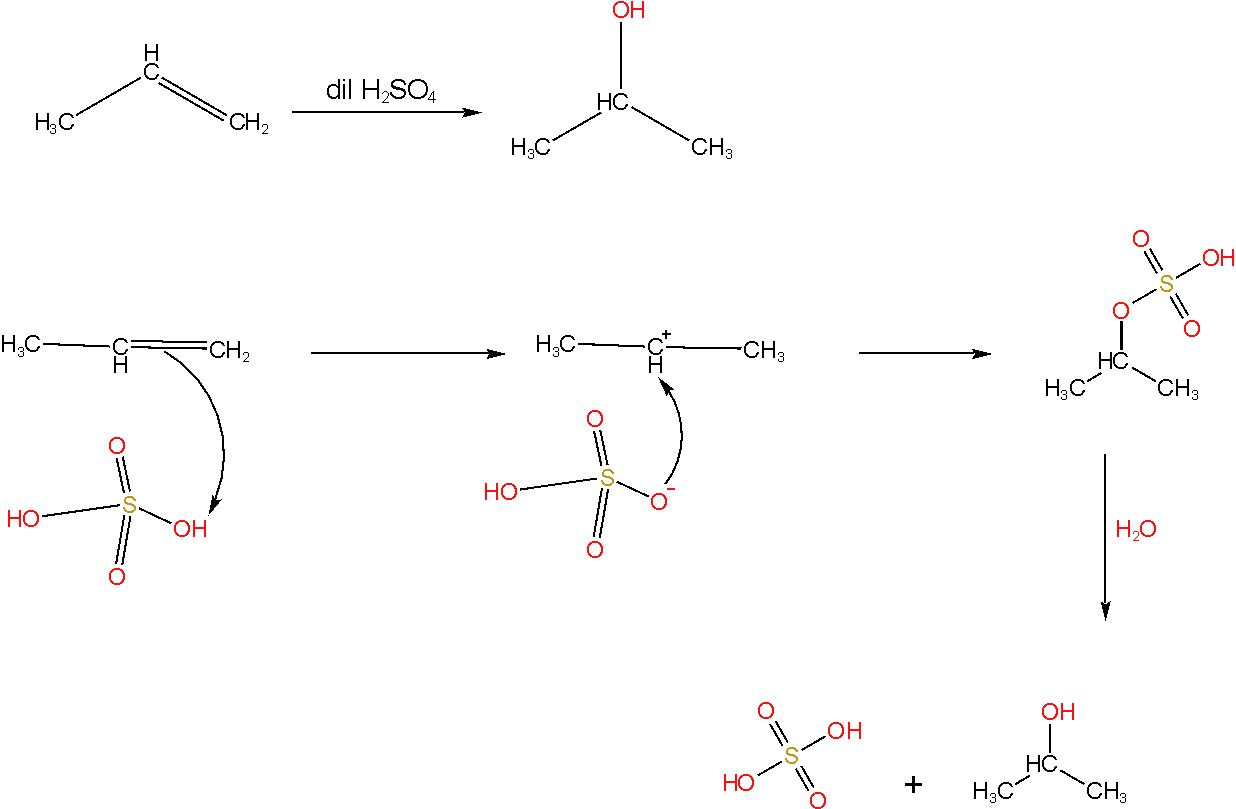
In the given reaction the nucleophile formed is the HSO4― ion which attacks the formed carbocation which contains the least number of hydrogen atoms. Thus on hydrolysis of propylene in presence of sulphuric acid isopropyl alcohol is formed.
Thus, Option (B) is correct
Note: In the given reaction sulphuric acid acts as a catalyst. During hydrolysis of alkene sometimes a mixture of alcohol also formed. The major alcohol formed will be from Markovnikov rule.
Complete Step by Step Answer:
On hydrolysis of alkene, the water molecule gets added to the alkene, and alcohol is formed. Since it is an addition reaction, this reaction will follow Markovnikov's law.
According to the addition mechanism of the alkene as the formation of carbocation takes place first the electrophile gets attached to the carbon which contains more no. of hydrogen atoms attached to the carbon and then the nucleophile gets attached to the carbon that contains less number of hydrogen atoms because according to markovnikov’s rule the nucleophile will attach with the carbon which contains less number of hydrogen.
In the addition reaction of alkene, the pi(π) bond between the two carbons of alkene breaks and they form two sigma bonds with two separate chemical species.
$CH_3-CH=CH_2 +\ \ dil.\ H_2SO_4\ \ \ \rightarrow CH_3-CH(OH)-CH_3$
Mechanism of the reaction:

In the given reaction the nucleophile formed is the HSO4― ion which attacks the formed carbocation which contains the least number of hydrogen atoms. Thus on hydrolysis of propylene in presence of sulphuric acid isopropyl alcohol is formed.
Thus, Option (B) is correct
Note: In the given reaction sulphuric acid acts as a catalyst. During hydrolysis of alkene sometimes a mixture of alcohol also formed. The major alcohol formed will be from Markovnikov rule.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




