
Nitrosobenzene can be isolated from nitrobenzene under
A. Metal and acid
B. Zn dust and $N{{H}_{4}}Cl$
C. Alkaline sodium arsenite
D. Cannot be isolated
Answer
233.1k+ views
Hint: Nitrosobenzene is formed electrochemically in a quantitative yield from nitrobenzene in an aprotic medium such as tetrahydrofuran in presence of acid. It can further be reduced to N-phenylhydroxylamine and aniline. These conversions are very rapid under an acidic medium.
Complete step by step solution:
The reduction of nitrobenzene to nitrosobenzene intermediate is thought to be primarily mediated by endogenous intestinal microbes. Aromatic nitroso compounds are very important in organic synthesis, for example, they are involved in modifying the energy level spacings and shift absorption from the ultraviolet into the blue-violet region radiating pale yellow color. This is a very useful shift in the characterization of the electron distribution.
It is found under investigation by metabolic studies that the reduction of nitrobenzene to aniline is a result of a three-step process, two electrons per step transfer with the intermediate nitrosobenzene and N-phenyl hydroxylamine. The overall three steps reaction sequences from nitrobenzene to aniline are shown below:
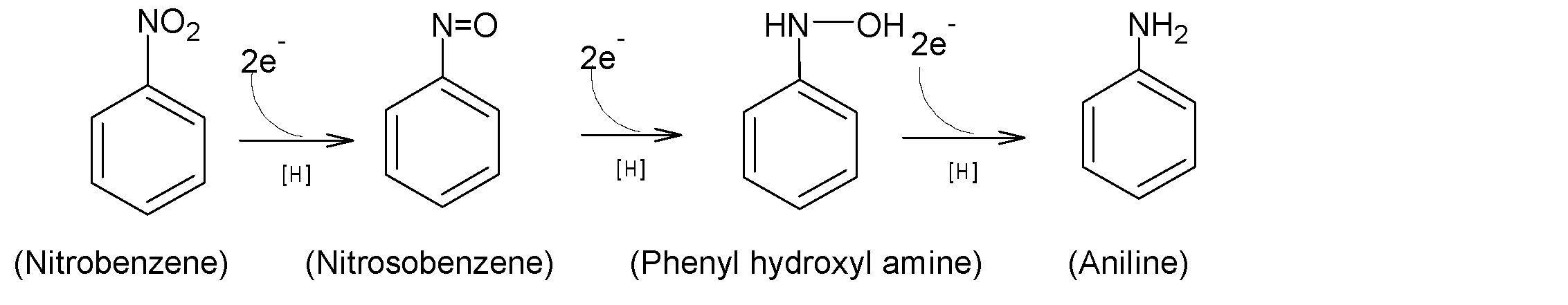
The presence of nitrosobenzene as an intermediate in the reduction of nitrobenzene can be done by trapping it with a suitable nucleophile. Sufficient stability of nitrosobenzene is detected for activating nitro compounds. Like nitro compounds, nitroso compounds are reduced but at a low potential.
Hence, nitrosobenzene cannot be distinguished directly from the reduction of nitrobenzene, as it undergoes further reduction very rapidly. Therefore nitrosobenzene can not be isolated from nitrobenzene.
Thus, option (D) is correct.
Note: In nitrosobenzene nitroso group ($-N=O$ ) is an ortho and para director for electrophilic substitution reaction and is an activator for nucleophilic substitution reactions. It is used to make various azo compounds in many synthetic reactions of organic chemistry where nitroso compounds act as active nucleophiles.
Complete step by step solution:
The reduction of nitrobenzene to nitrosobenzene intermediate is thought to be primarily mediated by endogenous intestinal microbes. Aromatic nitroso compounds are very important in organic synthesis, for example, they are involved in modifying the energy level spacings and shift absorption from the ultraviolet into the blue-violet region radiating pale yellow color. This is a very useful shift in the characterization of the electron distribution.
It is found under investigation by metabolic studies that the reduction of nitrobenzene to aniline is a result of a three-step process, two electrons per step transfer with the intermediate nitrosobenzene and N-phenyl hydroxylamine. The overall three steps reaction sequences from nitrobenzene to aniline are shown below:

The presence of nitrosobenzene as an intermediate in the reduction of nitrobenzene can be done by trapping it with a suitable nucleophile. Sufficient stability of nitrosobenzene is detected for activating nitro compounds. Like nitro compounds, nitroso compounds are reduced but at a low potential.
Hence, nitrosobenzene cannot be distinguished directly from the reduction of nitrobenzene, as it undergoes further reduction very rapidly. Therefore nitrosobenzene can not be isolated from nitrobenzene.
Thus, option (D) is correct.
Note: In nitrosobenzene nitroso group ($-N=O$ ) is an ortho and para director for electrophilic substitution reaction and is an activator for nucleophilic substitution reactions. It is used to make various azo compounds in many synthetic reactions of organic chemistry where nitroso compounds act as active nucleophiles.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
Understanding Average and RMS Value in Electrical Circuits

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Understanding Elastic Collisions in Two Dimensions

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

JEE Main Syllabus 2026: Download Detailed Subject-wise PDF

Other Pages
Alcohol Phenol and Ether Class 12 Chemistry Chapter 7 CBSE Notes - 2025-26

Haloalkanes and Haloarenes Class 12 Chemistry Chapter 6 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Understanding Collisions: Types and Examples for Students




