
$\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ and $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}\left( ONO \right) \right]C{{l}_{2}}$ are related to each other as:
A) Geometrical isomers
B) Optical isomers
C) Linkage isomers
D) Coordination isomers
Answer
233.1k+ views
Hint: In some coordination complexes, isomerism is observed when a ligand is bonded to the central atom through two or more than two atoms of the same ligand. Some ligands (like $N{{O}_{2}}$) consist of more than one atom which can act as the donor atom and can donate the electron pair to the central metal atom.
Complete answer:Linkage isomers: The coordination compounds which consist of the same composition of the ligands but the central metal ion is connected to different atoms of the same ligand. Those coordination compounds are known as Linkage isomers. As the bonding atom is different in both complexes, it is categorized under Structural isomerism.
For example Thiocyanate group i.e., $SC{{N}^{-}}$ can be bonded to the central metal atom through sulphur as well as nitrogen due to the availability of the lone pair of electrons on both atoms.
Some of the ligands that bond with central metal ions to show linkage isomerism are as follows:
1. Thiocyanate ($SC{{N}^{-}}$) in which the central metal ion is bonded to sulphur atom and Isothiocyanate ($NC{{S}^{-}}$) in which the central metal ion is bonded to the nitrogen atom.
2. Nitrite group can be bonded in two ways as $NO_{2}^{-}$ and $ON{{O}^{-}}$
In the given question, the complex given are $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ and $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}\left( ONO \right) \right]C{{l}_{2}}$which can be structurally represented as follows:
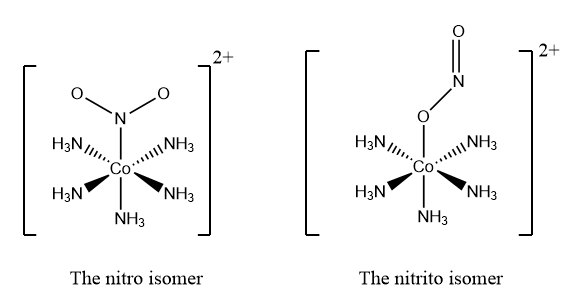
When the nitrogen atom is bonded to the central metal ion, then the complex is named as a nitro complex whereas when the oxygen atom donates its lone pair of electrons to cobalt, then the complex is named as nitrito complex.
Hence, the given complexes are related to each other by linkage isomerism. Therefore, option (C) i.e., linkage isomerism is the correct answer.
Note: It is important to note that in the linkage isomerism, the formula and composition of the complex remain the same but the properties of the complex differ from each other. Also, a change in the name of denoting the ligand is also observed (nitro and nitrito).
Complete answer:Linkage isomers: The coordination compounds which consist of the same composition of the ligands but the central metal ion is connected to different atoms of the same ligand. Those coordination compounds are known as Linkage isomers. As the bonding atom is different in both complexes, it is categorized under Structural isomerism.
For example Thiocyanate group i.e., $SC{{N}^{-}}$ can be bonded to the central metal atom through sulphur as well as nitrogen due to the availability of the lone pair of electrons on both atoms.
Some of the ligands that bond with central metal ions to show linkage isomerism are as follows:
1. Thiocyanate ($SC{{N}^{-}}$) in which the central metal ion is bonded to sulphur atom and Isothiocyanate ($NC{{S}^{-}}$) in which the central metal ion is bonded to the nitrogen atom.
2. Nitrite group can be bonded in two ways as $NO_{2}^{-}$ and $ON{{O}^{-}}$
In the given question, the complex given are $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}N{{O}_{2}} \right]C{{l}_{2}}$ and $\left[ Co{{\left( N{{H}_{3}} \right)}_{5}}\left( ONO \right) \right]C{{l}_{2}}$which can be structurally represented as follows:

When the nitrogen atom is bonded to the central metal ion, then the complex is named as a nitro complex whereas when the oxygen atom donates its lone pair of electrons to cobalt, then the complex is named as nitrito complex.
Hence, the given complexes are related to each other by linkage isomerism. Therefore, option (C) i.e., linkage isomerism is the correct answer.
Note: It is important to note that in the linkage isomerism, the formula and composition of the complex remain the same but the properties of the complex differ from each other. Also, a change in the name of denoting the ligand is also observed (nitro and nitrito).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




