
When ethyl iodide is heated with silver nitrate, the product obtained is
A. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{Ag}}\]
B.\[{\rm{Ag}} - {\rm{O}} - {\rm{N}}{{\rm{O}}_{\rm{2}}}\]
C.\[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{O}} - {\rm{N}}{{\rm{O}}_{\rm{2}}}\]
D. \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{I}} - {\rm{N}}{{\rm{O}}_{\rm{2}}}\]
Answer
233.1k+ views
Hint: Silver nitrate is an important organic compound used as an antiseptic and in many chemical reactions. The chemical symbol of silver nitrate is \[{\rm{AgN}}{{\rm{O}}_{\rm{3}}}\] . It is also used in the industrial preparation of many salts of silver.
Complete Step by Step Solution:
Let’s understand the reaction of ethyl iodide and silver nitrate. In the first step, dissociation of bonds of C-I and Ag-N occurs.
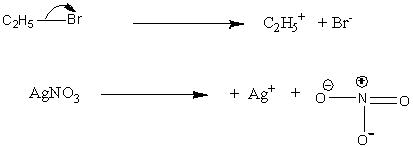
Image: Reaction of ethyl iodide and silver nitrate
In the second step, the nitrate ion attacks the carbocation and the product forms.
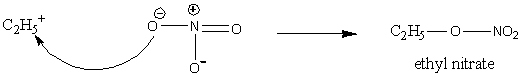
Image: Nucleophilic attack of nitrate ion
Therefore, the product formed in the reaction of ethyl iodide and silver nitrate is ethyl nitrate, \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{O}} - {\rm{N}}{{\rm{O}}_{\rm{2}}}\].
Hence, the option C is right
Additional Information: Let’s discuss ethyl iodide in detail. Ethyl iodide is also chemically known as iodoethane. It is a chemical compound of no colour and flammable properties. The chemical symbol of ethyl iodide is \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{I}}\]. The preparation of ethyl iodide is done by heating ethanol with phosphorus and iodine. The decomposition of iodoethane or ethyl iodide occurs when it comes in contact with air or light.
Note: There are many uses for silver nitrate in our everyday life. Silver nitrate is useful in treating wounds. It is used to treat bone ulcers, burns, and acute wounds. In the laboratory, Silver nitrate is a useful reagent because of its role in the identification of halogens, such as bromine, chlorine etc.
Complete Step by Step Solution:
Let’s understand the reaction of ethyl iodide and silver nitrate. In the first step, dissociation of bonds of C-I and Ag-N occurs.

Image: Reaction of ethyl iodide and silver nitrate
In the second step, the nitrate ion attacks the carbocation and the product forms.
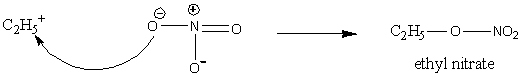
Image: Nucleophilic attack of nitrate ion
Therefore, the product formed in the reaction of ethyl iodide and silver nitrate is ethyl nitrate, \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{O}} - {\rm{N}}{{\rm{O}}_{\rm{2}}}\].
Hence, the option C is right
Additional Information: Let’s discuss ethyl iodide in detail. Ethyl iodide is also chemically known as iodoethane. It is a chemical compound of no colour and flammable properties. The chemical symbol of ethyl iodide is \[{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{I}}\]. The preparation of ethyl iodide is done by heating ethanol with phosphorus and iodine. The decomposition of iodoethane or ethyl iodide occurs when it comes in contact with air or light.
Note: There are many uses for silver nitrate in our everyday life. Silver nitrate is useful in treating wounds. It is used to treat bone ulcers, burns, and acute wounds. In the laboratory, Silver nitrate is a useful reagent because of its role in the identification of halogens, such as bromine, chlorine etc.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




