
Diamond is the hardest allotrope of carbon. Give reason for its hardness.
Answer
601.5k+ views
Hint: Carbon has well known allotropes like graphite and diamond. It is very interesting to know that both are made up of the same element, carbon, yet diamond is the hardest known mineral whereas graphite is very soft. The reason lies in the physical structure of the two.
Complete answer:
Allotropes of an element are the structurally different forms of the same element. They have the same atoms of the element but the structures in which these atoms are arranged vary greatly. This results in various physical forms of the same element. Allotropes differ in physical as well as chemical properties even though they are composed of molecules of the same element. The structure of graphite and diamond are shown below;
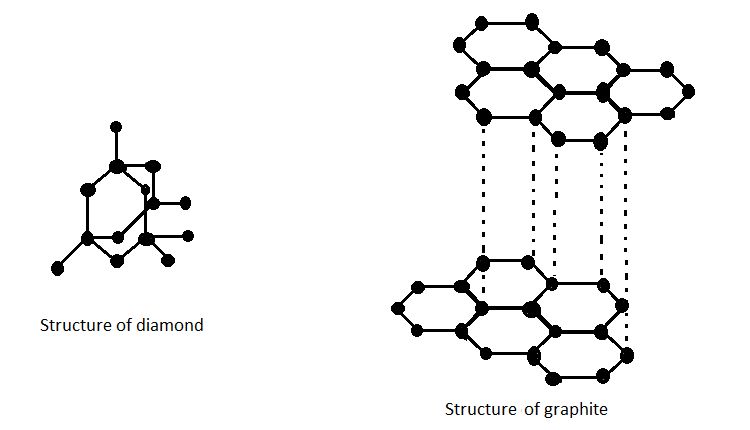
Each carbon atom in a diamond is covalently bonded to four other carbons forming a tetrahedral structure. These tetrahedrons together form a 3-dimensional network of carbon rings. This stable network of covalent bonds and hexagonal rings makes diamond the hardest allotrope of carbon and also the hardest mineral. Graphite has covalent structure where each carbon atom is joined to three carbon atoms by covalent bond. The carbon atoms form layers with a hexagonal arrangement of atoms.
Carbon is known to form many allotropes due to its valency. There are 8 allotropes of carbon, the most common being diamond and graphite.
Note: Contrast to diamond, graphite is a very soft allotrope of carbon. It is dull and dark unlike diamond which is known for its shining and luster. However, graphite can conduct electricity and diamond cannot. The reason lies again in the difference of structure between the two.
Complete answer:
Allotropes of an element are the structurally different forms of the same element. They have the same atoms of the element but the structures in which these atoms are arranged vary greatly. This results in various physical forms of the same element. Allotropes differ in physical as well as chemical properties even though they are composed of molecules of the same element. The structure of graphite and diamond are shown below;

Each carbon atom in a diamond is covalently bonded to four other carbons forming a tetrahedral structure. These tetrahedrons together form a 3-dimensional network of carbon rings. This stable network of covalent bonds and hexagonal rings makes diamond the hardest allotrope of carbon and also the hardest mineral. Graphite has covalent structure where each carbon atom is joined to three carbon atoms by covalent bond. The carbon atoms form layers with a hexagonal arrangement of atoms.
Carbon is known to form many allotropes due to its valency. There are 8 allotropes of carbon, the most common being diamond and graphite.
Note: Contrast to diamond, graphite is a very soft allotrope of carbon. It is dull and dark unlike diamond which is known for its shining and luster. However, graphite can conduct electricity and diamond cannot. The reason lies again in the difference of structure between the two.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




