
Compound given below is: -
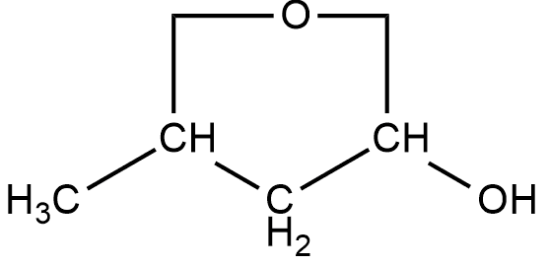 A.Tautomer of $3$- ox butanol and also show structural isomerism
A.Tautomer of $3$- ox butanol and also show structural isomerism
B.Tautomer of butanoic acid
C.Compound does not show any isomerism
D.The compound can show structural isomers except tautomerism

Answer
517.5k+ views
Hint: Tautomers are defined as two compounds existing together existing in equilibrium with each other. These two compounds need to be isomers of each other. This happens by the migration of one of the elements to migrate in between the compound only.
Complete answer:
Tautomers are defined as the two compounds existing together which are isomers of each other.
First let’s understand what isomers mean, isomers are defined as the pair of compounds which have the same formula but the arrangement of the two compounds is very different from each other.
First let’s name the carbons from right to left
If we break the chain then we are going two have two pairs of compounds with the same formula and yne of them would have a hydroxide ion while the other carbon would have hydrogen ion attached to it.
So, this gives us two orientations, first is the one in which the first carbon has the hydroxide ion and the third carbon would have hydrogen ion. too
The second orientation is in which the first carbon has the hydrogen ion and the third carbon would be hydroxide ion.
But the hydroxide carbon is So, far away from the hydrogen carbon, they have a carbon between them, that’s why there is too much hindrance for any reaction to happen.
Therefore, going by the definition of tautomer, that is they exist in equilibrium with each other, but here they both are stable compounds alone.
But they are isomers as they have the same formula
And hence the correct answer is option D i.e The compound can show structural isomers except tautomers.
Note:
Structural isomers are those which have the same formula, but different orientation. Structural isomers are further divided into chain isomers, position isomers, and functional isomers. In one the chain is different, position of the functional group is different and the functional group is different, respectively.
Complete answer:
Tautomers are defined as the two compounds existing together which are isomers of each other.
First let’s understand what isomers mean, isomers are defined as the pair of compounds which have the same formula but the arrangement of the two compounds is very different from each other.
First let’s name the carbons from right to left
If we break the chain then we are going two have two pairs of compounds with the same formula and yne of them would have a hydroxide ion while the other carbon would have hydrogen ion attached to it.
So, this gives us two orientations, first is the one in which the first carbon has the hydroxide ion and the third carbon would have hydrogen ion. too
The second orientation is in which the first carbon has the hydrogen ion and the third carbon would be hydroxide ion.
But the hydroxide carbon is So, far away from the hydrogen carbon, they have a carbon between them, that’s why there is too much hindrance for any reaction to happen.
Therefore, going by the definition of tautomer, that is they exist in equilibrium with each other, but here they both are stable compounds alone.
But they are isomers as they have the same formula
And hence the correct answer is option D i.e The compound can show structural isomers except tautomers.
Note:
Structural isomers are those which have the same formula, but different orientation. Structural isomers are further divided into chain isomers, position isomers, and functional isomers. In one the chain is different, position of the functional group is different and the functional group is different, respectively.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




