
Colour of CuO is
A. Black
B. Blue
C. Green
D. Red
Answer
233.1k+ views
Hint: In order to answer the question , we need to have a detailed idea about the structure of CuO. We should also be familiar with the physical and chemical properties of CuO.
Complete step-by-step answer:
Let us have a look into the structure of CuO.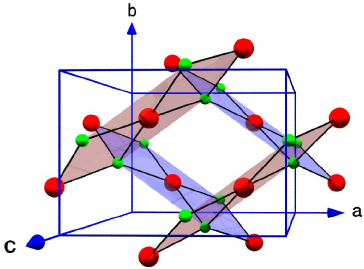
The red structures are the Copper atoms and the green structures are the oxygen atoms.
The atomic number of copper is 29. And that of oxygen is 8.
So the electronic configurations of both the elements are given below:
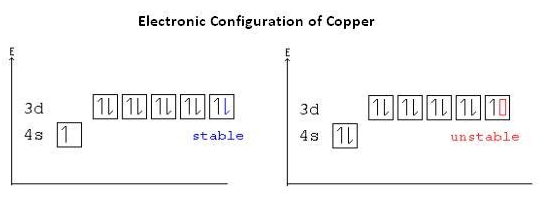
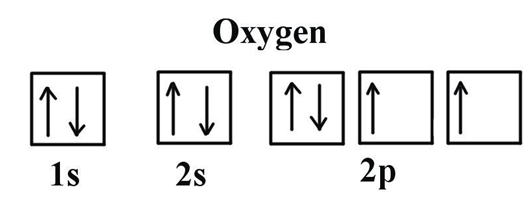
The main factor to occur the colour of the compounds of d block, is having the d orbitals with an incomplete electron filling in the middle atom.
There is an inequality of energy, among those d orbitals. Therefore d-d electrons exchange among those orbitals by absorbing energy. The amount of energy consumption causes the colour of the compound. Here, different amounts of energy need to exchange different numbers of electrons. Thereby the wavelengths absorbed in the process changes, and consequently different colours occur.
The colour of CuO is known to be Black. So the correct option is Option A.
Note: We should be knowing the few uses of CuO. So here are some uses of CuO listed below:
Used in the welding process along with various copper alloys.
Used as a precursor in various copper containing products like wood preservatives and ceramics.
Some properties of CuO are:
Super Thermal Conductivity.
CuO shows photovoltaic properties.
It has high stability and high antimicrobial activity.
Complete step-by-step answer:
Let us have a look into the structure of CuO.

The red structures are the Copper atoms and the green structures are the oxygen atoms.
The atomic number of copper is 29. And that of oxygen is 8.
So the electronic configurations of both the elements are given below:


The main factor to occur the colour of the compounds of d block, is having the d orbitals with an incomplete electron filling in the middle atom.
There is an inequality of energy, among those d orbitals. Therefore d-d electrons exchange among those orbitals by absorbing energy. The amount of energy consumption causes the colour of the compound. Here, different amounts of energy need to exchange different numbers of electrons. Thereby the wavelengths absorbed in the process changes, and consequently different colours occur.
The colour of CuO is known to be Black. So the correct option is Option A.
Note: We should be knowing the few uses of CuO. So here are some uses of CuO listed below:
Used in the welding process along with various copper alloys.
Used as a precursor in various copper containing products like wood preservatives and ceramics.
Some properties of CuO are:
Super Thermal Conductivity.
CuO shows photovoltaic properties.
It has high stability and high antimicrobial activity.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




