
By which of the following, poisoning of lead in the body can be removed?
A.\[Pt\]
B.\[EDTA\]
C.\[O{x^{2 - }}\]
D.\[Pn\]
Answer
517.5k+ views
Hint: The chemical compound which is used in removing the poisoning of lead is a ligand. It is a hexadentate ligand i.e., it consists of four oxygen atoms and two nitrogen atoms which acts as donor atoms. This compound is used in the labs for volumetric titrations.
Complete answer:
Poisoning of lead is a type of metal poisoning caused due to the presence of excess of lead in the human body. The several symptoms of lead poisoning are memory loss, abdominal pain, headache, etc. A person can suffer lead poisoning due to continuous exposure to polluted water, air and some cosmetic products also contain lead particles.
Hence, for removing excess lead in the body we need a ligand which can form complex and then can be easily removed. The ligand used for this purpose is EDTA.
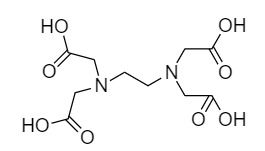
EDTA is a hexadentate ligand and its full form is ethylene diamine tetra-acetic acid. Its molecular formula is \[{C_{10}}{H_{16}}{N_2}{O_8}\] and structurally it is represented as follows:
Pt i.e., Platinum metal is used as anti-cancer agent in the form of cis- Platin.
\[Pn\] i.e., penicillin is used for curing copper poisoning in the form of penicillamine.
\[O{x^{2 - }}\]i.e., oxalate ion is widely used for rust removal because it forms water soluble derivatives on reacting with ferric ions.
Hence, it is concluded that with the help of EDTA, the poisoning of lead in the body can be removed.
Thus, option (B) is the correct answer.
Note:
EDTA is also a chelating agent as it forms a ring while forming dative bonds with the metal. Thus, the process of removing lead poisoning from the body is also known as chelation therapy. The chelating factor provides extra stability to the complexes.
Complete answer:
Poisoning of lead is a type of metal poisoning caused due to the presence of excess of lead in the human body. The several symptoms of lead poisoning are memory loss, abdominal pain, headache, etc. A person can suffer lead poisoning due to continuous exposure to polluted water, air and some cosmetic products also contain lead particles.
Hence, for removing excess lead in the body we need a ligand which can form complex and then can be easily removed. The ligand used for this purpose is EDTA.
EDTA is a hexadentate ligand and its full form is ethylene diamine tetra-acetic acid. Its molecular formula is \[{C_{10}}{H_{16}}{N_2}{O_8}\] and structurally it is represented as follows:

Pt i.e., Platinum metal is used as anti-cancer agent in the form of cis- Platin.
\[Pn\] i.e., penicillin is used for curing copper poisoning in the form of penicillamine.
\[O{x^{2 - }}\]i.e., oxalate ion is widely used for rust removal because it forms water soluble derivatives on reacting with ferric ions.
Hence, it is concluded that with the help of EDTA, the poisoning of lead in the body can be removed.
Thus, option (B) is the correct answer.
Note:
EDTA is also a chelating agent as it forms a ring while forming dative bonds with the metal. Thus, the process of removing lead poisoning from the body is also known as chelation therapy. The chelating factor provides extra stability to the complexes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




