
How many bonds are present in \[S - S\] bonds in pyrosulphuric acid (oleum)?
Answer
521.1k+ views
Hint: Pyrosulphuric acid or disulfuric acid is the main constituent of fuming sulfuric acid. It is a solution of sulfur trioxide $(S{O_3})$ in anhydrous sulfuric acid. Pyrosulphuric acid is a colourless liquid that fumes in moist air due to the reaction between $S{O_3}$ and ${H_2}O$, which leads to the formation of poorly volatile ${H_2}S{O_4}$. It is also used for sulfation of organic compounds.
Complete answer:
We can determine the number of \[S - S\] bonds present in pyrosulfuric acid (oleum) by taking a look at its structure.
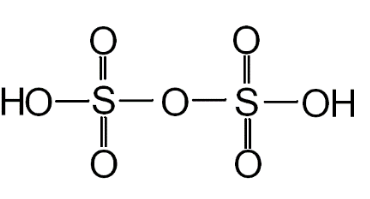
Fig: Structure of pyrosulfuric acid (oleum).
From the above structure we can easily figure out that there are no \[S - S\] bonds present in oleum.
Therefore, the number of bonds present in \[S - S\] bonds in pyrosulfuric acid (oleum) is zero.
Note:
Oleum is a harsh reagent and is highly corrosive. Due to the high enthalpy of oleum it is used as an important intermediate in the manufacture of sulfuric acid. It is also used as a reagent in the secondary nitration of nitrobenzene.
Complete answer:
We can determine the number of \[S - S\] bonds present in pyrosulfuric acid (oleum) by taking a look at its structure.

Fig: Structure of pyrosulfuric acid (oleum).
From the above structure we can easily figure out that there are no \[S - S\] bonds present in oleum.
Therefore, the number of bonds present in \[S - S\] bonds in pyrosulfuric acid (oleum) is zero.
Note:
Oleum is a harsh reagent and is highly corrosive. Due to the high enthalpy of oleum it is used as an important intermediate in the manufacture of sulfuric acid. It is also used as a reagent in the secondary nitration of nitrobenzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




