
Anthracene can be electrolytically oxidised to anthraquinone. What mass of anthraquinone can be produced by the passage of 1 ampere current for an hour at 100% efficiency?

A. A. 3.654 g
B. 2 g
C. 12 g
D. 1.2932 g
Answer
232.5k+ views
Hint: In this question, anthracene is getting oxidised to anthraquinone electrolytically. Therefore we will use the Faraday’s laws of electrolysis in order to determine the amount of anthraquinone produced.
Complete step by step solution:
In this question, we will use Faraday’s first law of electrolysis which states that the amount of a substance that gets deposited on an electrode during electrolysis is directly proportional to the amount of charge passed through the electrolytic solution i.e.
$ { mass\quad of\quad substance\quad deposited\quad \propto \quad Amount\quad of\quad charge }$
$ \Rightarrow m=ZQ$
Where ‘m’ is the mass of the substance deposited, ‘Q’ is the amount of the charge passed through the solution and ‘Z’ is the proportionality constant; known as the electro-chemical equivalent of the substance. ‘Z’ will be equal to the amount of the substance deposited when a charge of 1 coulomb is passed through the solution.
Let us examine the reaction given in the question:
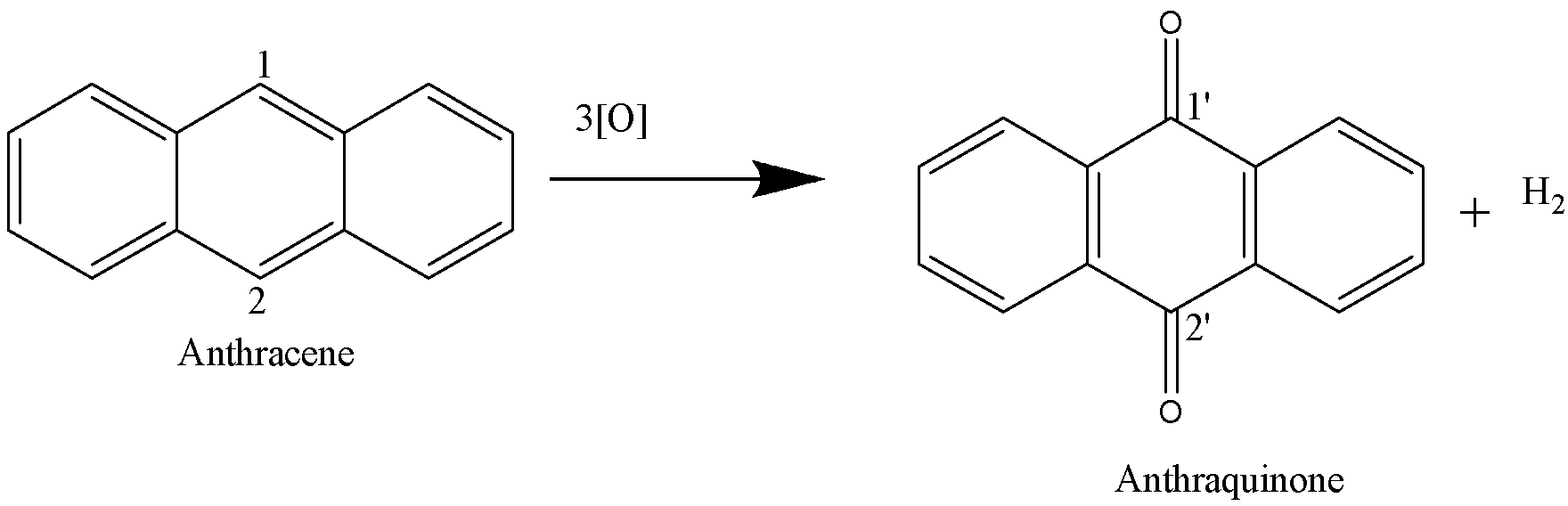
In order to find the total amount of charge required to convert 1 mole of anthracene to 1 mole of anthraquinone, we need to find the valency factor which is the total change in oxidation number that the atoms in a species undergo per molecule of the species.
In the above reaction, the carbon atoms labelled as ‘1’ and ‘2’ are undergoing a change in their oxidation state. The oxidation number of carbon ‘1’ in anthracene is -1 whereas the oxidation number of the carbon ‘1’’ in anthraquinone is +2. The total change in oxidation number is +3 i.e.[+2-(-1)]=+3. Since the total number of carbon atoms that are undergoing the change in oxidation number in anthracene is two, therefore a total of 6 electrons ($2\times (3)=6$) are required to convert 1 molecule of anthracene into 1 molecule of anthraquinone. Therefore a total of 6 moles of electrons will be required to produce 1 mole of anthraquinone from 1 mole of anthracene. 6 moles of electrons will carry 6 Farad of electric charge (1F=96500 C/mol). The Molar mass of anthraquinone is 208 g/mol. Therefore by using the Faraday’s first law of electrolysis:
$ \begin{matrix} 208 g/mol \\ Molar\quad\ mass\ of\ anthraquinone \end{matrix}=Z\times 6\times 96500\quad C/mol$
$ \Rightarrow Z=\cfrac { 208 g/mol }{ 6\times 96500 C/mol } $
Now, when a current of 1 ampere is passed through anthracene for 1 hour, it produces a total charge of:
$ Q=I\times t$ ; where ‘Q’ is the total charge, ‘I’ is the current passed and ‘t’ is the time for which the current is passed.
$ \Rightarrow Q=1A\times 60\times 60s$
Therefore:
$ \begin{matrix} m \\ Mass\ of\ Anthraquinone \end{matrix}=\begin{matrix} \cfrac { 208 g/mol }{ 6\times 96500 C/mol } \\ (Z) \end{matrix}\times \begin{matrix} 1A \\ I \end{matrix}\times \begin{matrix} 60\times 60s \\ t \end{matrix}$
$\begin{matrix} m \\ Mass\ of\ Anthraquinone \end{matrix}=1.2932 g$
Therefore, the correct answer is (D) 1.2932 g.
Note: Always remember that the valency factor in this case will be 6 and not 3 since a total of 2 carbon atoms in anthracene are undergoing a change in their oxidation state from -1 in anthracene to +2 in anthraquinone.
Complete step by step solution:
In this question, we will use Faraday’s first law of electrolysis which states that the amount of a substance that gets deposited on an electrode during electrolysis is directly proportional to the amount of charge passed through the electrolytic solution i.e.
$ { mass\quad of\quad substance\quad deposited\quad \propto \quad Amount\quad of\quad charge }$
$ \Rightarrow m=ZQ$
Where ‘m’ is the mass of the substance deposited, ‘Q’ is the amount of the charge passed through the solution and ‘Z’ is the proportionality constant; known as the electro-chemical equivalent of the substance. ‘Z’ will be equal to the amount of the substance deposited when a charge of 1 coulomb is passed through the solution.
Let us examine the reaction given in the question:

In order to find the total amount of charge required to convert 1 mole of anthracene to 1 mole of anthraquinone, we need to find the valency factor which is the total change in oxidation number that the atoms in a species undergo per molecule of the species.
In the above reaction, the carbon atoms labelled as ‘1’ and ‘2’ are undergoing a change in their oxidation state. The oxidation number of carbon ‘1’ in anthracene is -1 whereas the oxidation number of the carbon ‘1’’ in anthraquinone is +2. The total change in oxidation number is +3 i.e.[+2-(-1)]=+3. Since the total number of carbon atoms that are undergoing the change in oxidation number in anthracene is two, therefore a total of 6 electrons ($2\times (3)=6$) are required to convert 1 molecule of anthracene into 1 molecule of anthraquinone. Therefore a total of 6 moles of electrons will be required to produce 1 mole of anthraquinone from 1 mole of anthracene. 6 moles of electrons will carry 6 Farad of electric charge (1F=96500 C/mol). The Molar mass of anthraquinone is 208 g/mol. Therefore by using the Faraday’s first law of electrolysis:
$ \begin{matrix} 208 g/mol \\ Molar\quad\ mass\ of\ anthraquinone \end{matrix}=Z\times 6\times 96500\quad C/mol$
$ \Rightarrow Z=\cfrac { 208 g/mol }{ 6\times 96500 C/mol } $
Now, when a current of 1 ampere is passed through anthracene for 1 hour, it produces a total charge of:
$ Q=I\times t$ ; where ‘Q’ is the total charge, ‘I’ is the current passed and ‘t’ is the time for which the current is passed.
$ \Rightarrow Q=1A\times 60\times 60s$
Therefore:
$ \begin{matrix} m \\ Mass\ of\ Anthraquinone \end{matrix}=\begin{matrix} \cfrac { 208 g/mol }{ 6\times 96500 C/mol } \\ (Z) \end{matrix}\times \begin{matrix} 1A \\ I \end{matrix}\times \begin{matrix} 60\times 60s \\ t \end{matrix}$
$\begin{matrix} m \\ Mass\ of\ Anthraquinone \end{matrix}=1.2932 g$
Therefore, the correct answer is (D) 1.2932 g.
Note: Always remember that the valency factor in this case will be 6 and not 3 since a total of 2 carbon atoms in anthracene are undergoing a change in their oxidation state from -1 in anthracene to +2 in anthraquinone.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




