
Among the following compounds nitrobenzene, benzene, aniline and phenol, the strongest basic behaviuor in acidic medium is exhibited by:
A. Phenol
B. Aniline
C. Nitrobenzene
D. Benzene
Answer
233.1k+ views
Hint: Basicity of species defines the basic character of the species. As the electron donating power of a species increases its basic character also increases. In acidic medium the basic character can be defined by electron donating power or hydrogen ion accepting power. It depends on several factors like- the groups attached, the resonance effect etc.
Complete step-by-step answer:Stability factor of base:
-As the tendency to participate in resonance increases the stability of the base also increases.
-A electron donating group attached to a species its basicity increases because the electron donating group donates its electron to benzene ring and hydrogen ion accepting tendency of the species increases.
-But an electron withdrawing group decreases the basicity of a species.
Stability comparison:
-Phenol or $C_6H_5OH$ can easily donate its hydrogen atom and acts as an acid.
-Aniline or $C_6H_5NH_2$ has electron donating $N$ atom attached with benzene and Nitrogen has a lone pair on it which it can donate to act as an base or accept protons to form ammonium ions.
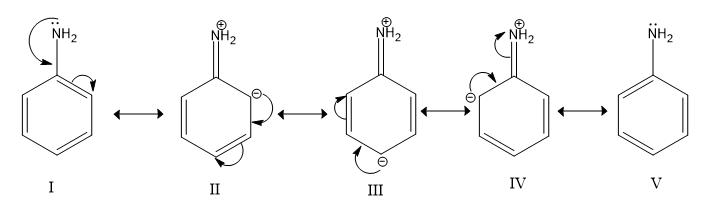
Image: Resonance of Aniline
-Nitrobenzene or $C_6H_5NO_2$ has an electron withdrawing nitro group attached with benzene which decreases its electron donating power and basic character decreases. The lone pair of electrons on N is not available for donation.
-Benzene can donate its electron pair and act as a moderately strong base.
Thus aniline is most basic in acidic solution.
Option ‘B’ is correct
Note: Basic character or basicity of a species defines the electron donating power. The acidic character or acidity of a species defines electron accepting power. Basicity increases with increase in ease of lone pair of electrons donation.
Complete step-by-step answer:Stability factor of base:
-As the tendency to participate in resonance increases the stability of the base also increases.
-A electron donating group attached to a species its basicity increases because the electron donating group donates its electron to benzene ring and hydrogen ion accepting tendency of the species increases.
-But an electron withdrawing group decreases the basicity of a species.
Stability comparison:
-Phenol or $C_6H_5OH$ can easily donate its hydrogen atom and acts as an acid.
-Aniline or $C_6H_5NH_2$ has electron donating $N$ atom attached with benzene and Nitrogen has a lone pair on it which it can donate to act as an base or accept protons to form ammonium ions.

Image: Resonance of Aniline
-Nitrobenzene or $C_6H_5NO_2$ has an electron withdrawing nitro group attached with benzene which decreases its electron donating power and basic character decreases. The lone pair of electrons on N is not available for donation.
-Benzene can donate its electron pair and act as a moderately strong base.
Thus aniline is most basic in acidic solution.
Option ‘B’ is correct
Note: Basic character or basicity of a species defines the electron donating power. The acidic character or acidity of a species defines electron accepting power. Basicity increases with increase in ease of lone pair of electrons donation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




