
How do amino acids behave in acidic and basic solutions?
Answer
528.3k+ views
Hint: Amino acid here in this, we will look at how amino acid behaves with acid and bases separately.
We know that amino acid contains a single group $N{H_2}$ and a single group \[ - COOH\]. It has a property of forming zwitterion ions, because of the presence of the above-mentioned group.
Complete answer:
It is basically the amino acid has both a basic amine group as well as an acidic carboxylic group
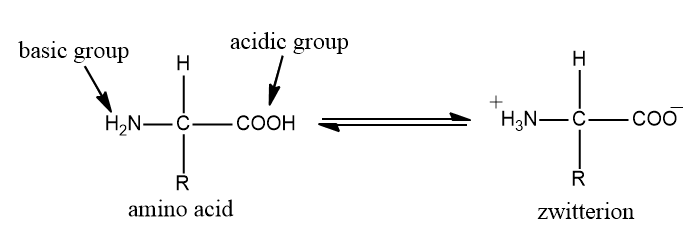
Adding base to amino acid solution
We will increase the pH of the solution of amino acid by adding hydroxide ions, the hydrogen ion will remove from the \[N{H_3}^ + \] group. If we run it under electrophoresis, it has negative charge.
Suppose we have an amino group under acidic condition and we slowly add alkali. So, when we add just the right amount of alkali, the more acidic hydrogen of the carboxylic group is removed instead of amino group hydrogen. And we get back zwitterion. the amino group will no longer have net positive or negative charge. This means, it wouldn’t move toward the cathode or anode during electrophoresis.
Amino in acidic condition,
if we decrease the pH by adding an acid to the solution, the \[ - COO\] part of zwitterion picks up the hydrogen ion. This time electrophoresis would move amino acid towards the cathode which is a negative electrode.
Note:
There is an internal transfer of a hydrogen ion from the carboxylic group to the amino group. This is the form that amino acids exist even in solid state. If you dissolve the amino acid in water, then this solution will contain this ion.
We know that amino acid contains a single group $N{H_2}$ and a single group \[ - COOH\]. It has a property of forming zwitterion ions, because of the presence of the above-mentioned group.
Complete answer:
It is basically the amino acid has both a basic amine group as well as an acidic carboxylic group

Adding base to amino acid solution
We will increase the pH of the solution of amino acid by adding hydroxide ions, the hydrogen ion will remove from the \[N{H_3}^ + \] group. If we run it under electrophoresis, it has negative charge.
Suppose we have an amino group under acidic condition and we slowly add alkali. So, when we add just the right amount of alkali, the more acidic hydrogen of the carboxylic group is removed instead of amino group hydrogen. And we get back zwitterion. the amino group will no longer have net positive or negative charge. This means, it wouldn’t move toward the cathode or anode during electrophoresis.
Amino in acidic condition,
if we decrease the pH by adding an acid to the solution, the \[ - COO\] part of zwitterion picks up the hydrogen ion. This time electrophoresis would move amino acid towards the cathode which is a negative electrode.
Note:
There is an internal transfer of a hydrogen ion from the carboxylic group to the amino group. This is the form that amino acids exist even in solid state. If you dissolve the amino acid in water, then this solution will contain this ion.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE

Define peptide linkage class 12 chemistry CBSE

Which compound gives positive iodoform test A2pentanone class 12 chemistry CBSE

Write the different structural and functional differences class 12 chemistry CBSE




