
How would you achieve the following conversions?
i) Aniline to Benzonitrile
ii) Nitrobenzene to phenol
iii) Ethyl amine to ethyl isonitrile
Answer
525.3k+ views
Hint: Electrophilic substitution reactions: These are the chemical reactions in which an electrophile attacks the carbon atom and replaces the functional group present on it. For the given conversions, we need to follow electrophilic reaction mechanisms.
Complete answer:
(a) Conversion of Aniline to benzonitrile: Aniline is converted to benzonitrile by diazotization reaction followed by Sandmeyer reaction. When activated aromatic compounds like aniline reacts with sodium nitrite in the presence of hydrochloric acid, then the formation of benzene diazonium chloride takes place which is when further reacted with copper cyanide, yields benzonitrile along with the removal of nitrogen gas and copper chloride.
Mechanism for the conversion is as follows:
Step-1: Formation of benzene diazonium chloride from aniline
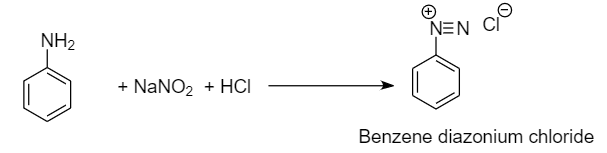
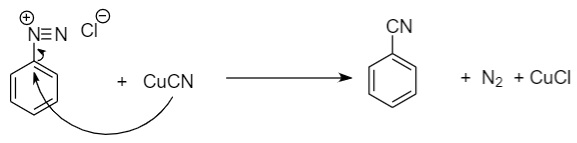
Step-2: Further reaction with \[CuCN\] to form benzonitrile.
(b) Conversion of nitrobenzene to phenol:
For the given conversion, first we need to convert nitrobenzene into aniline by reducing it in the presence of tin and hydrochloric acid. Then aniline undergoes diazotization reaction to form benzene diazonium chloride which on further reaction with water at warm conditions, gives phenol and removal of nitrogen gas and hydrochloric acid takes place.
Mechanism for the conversion is as follows:
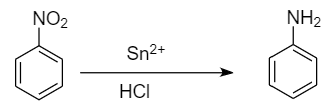
Step-1: Reduction of nitrobenzene to aniline:
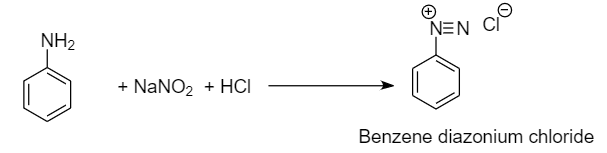
Step-2: Formation of benzene diazonium chloride from aniline:
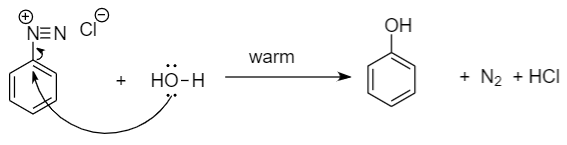
Step-3: Further reaction of benzene diazonium chloride with water to yield phenol:
(c) Conversion of ethyl amine to ethyl isonitrile:
The given conversion follows the mechanism for carbylamine reaction in which the given primary amine reacts with the carbene to form alkyl isonitrile in the presence of potassium hydroxide. Mechanism for the reaction is as follows:
Step-1: Dissociation of potassium hydroxide:
$KOH\rightleftharpoons {{K}^{+}}+O{{H}^{-}}$
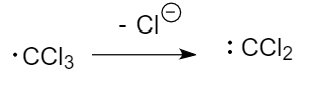
Step-2: Formation of trichlorocarbene from chloroform:

Step-3: Formation of dichlorocarbene:
Step-4: Reaction of dichlorocarbene with ethyl amine:

Step-5: Further reaction with potassium hydroxide to give ethyl isonitrile:
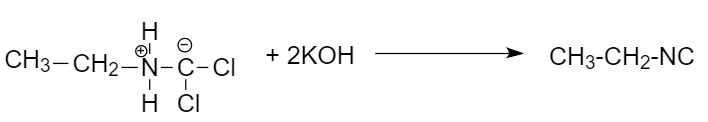
Hence, all the given conversions are followed by electrophilic substitution reaction.
Note:
Carbene is an intermediate which consists of a neutral carbon atom with two unshared valence electrons and due to this, it acts as an electrophile in many organic reactions. Carbene is of two types, triplet carbene and singlet carbene but triplet carbene is known to be more stable than that of singlet carbene.
Complete answer:
(a) Conversion of Aniline to benzonitrile: Aniline is converted to benzonitrile by diazotization reaction followed by Sandmeyer reaction. When activated aromatic compounds like aniline reacts with sodium nitrite in the presence of hydrochloric acid, then the formation of benzene diazonium chloride takes place which is when further reacted with copper cyanide, yields benzonitrile along with the removal of nitrogen gas and copper chloride.
Mechanism for the conversion is as follows:
Step-1: Formation of benzene diazonium chloride from aniline

Step-2: Further reaction with \[CuCN\] to form benzonitrile.

(b) Conversion of nitrobenzene to phenol:
For the given conversion, first we need to convert nitrobenzene into aniline by reducing it in the presence of tin and hydrochloric acid. Then aniline undergoes diazotization reaction to form benzene diazonium chloride which on further reaction with water at warm conditions, gives phenol and removal of nitrogen gas and hydrochloric acid takes place.
Mechanism for the conversion is as follows:
Step-1: Reduction of nitrobenzene to aniline:

Step-2: Formation of benzene diazonium chloride from aniline:

Step-3: Further reaction of benzene diazonium chloride with water to yield phenol:

(c) Conversion of ethyl amine to ethyl isonitrile:
The given conversion follows the mechanism for carbylamine reaction in which the given primary amine reacts with the carbene to form alkyl isonitrile in the presence of potassium hydroxide. Mechanism for the reaction is as follows:
Step-1: Dissociation of potassium hydroxide:
$KOH\rightleftharpoons {{K}^{+}}+O{{H}^{-}}$
Step-2: Formation of trichlorocarbene from chloroform:

Step-3: Formation of dichlorocarbene:

Step-4: Reaction of dichlorocarbene with ethyl amine:

Step-5: Further reaction with potassium hydroxide to give ethyl isonitrile:
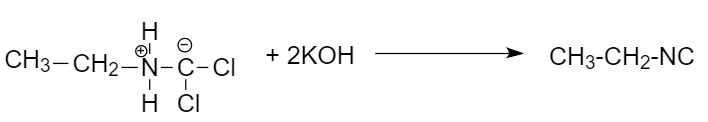
Hence, all the given conversions are followed by electrophilic substitution reaction.
Note:
Carbene is an intermediate which consists of a neutral carbon atom with two unshared valence electrons and due to this, it acts as an electrophile in many organic reactions. Carbene is of two types, triplet carbene and singlet carbene but triplet carbene is known to be more stable than that of singlet carbene.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




