
A compound of formula $ {A_2}{B_3} $ has the hcp lattice. Which atom forms the hcp lattice and what fraction of tetrahedral voids is occupied by the other atoms?
(A) $ {\text{hcp lattice - A, }}\dfrac{2}{3}{\text{ Tetrahedral voids - B}} $
(B) $ {\text{hcp lattice - B, }}\dfrac{1}{3}{\text{ Tetrahedral voids - A}} $
(C) $ {\text{hcp lattice - B, }}\dfrac{2}{3}{\text{ Tetrahedral voids - A}} $
(D) $ {\text{hcp lattice - A, }}\dfrac{1}{3}{\text{ Tetrahedral voids - B}} $
Answer
520.8k+ views
Hint :The hcp structure of a unit cell has three types of atoms in three layers. There are twelve atoms at the corners of the hexagon. Three atoms are present in between the top and bottom face of the hexagon, in alternate vertical faces. These atoms are present inside the face so that they cannot be shared by other cells.
Complete Step By Step Answer:
the structure of hcp unit cell is shown below:
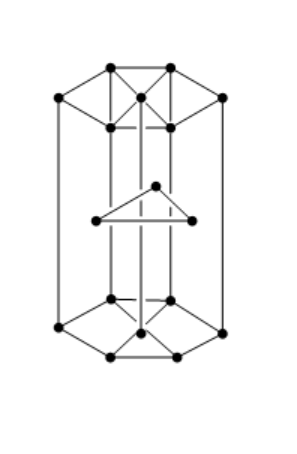
Let us consider that $ B $ will produce the hcp lattice.
Total number of atoms in $ B = 6 $
Tetrahedral void occupied $ = 2N $
So, $ N = 6 $
Tetrahedral void= $ 2 \times 6 = 12 $
Now, the number of atoms in A $ = \dfrac{1}{3} \times {\text{Tetrahedral void}} $
$ = \dfrac{1}{3} \times 12 $
$ = 4 $
So, the compound will be $ {A_4}{B_6} $ which can be simplified and written as $ {A_2}{B_3} $
Therefore, option B is the correct answer.
Note :
The closest packing of spheres in two dimensions has a hexagonal symmetry in which every sphere has six nearest neighbors. In hcp, each atom has twelve nearest neighbours. Consider an ideal structure, then the distance between the planes is $ 1.633a $ , where $ a $ is the distance between the atoms of the unit cell.
Complete Step By Step Answer:
the structure of hcp unit cell is shown below:

Let us consider that $ B $ will produce the hcp lattice.
Total number of atoms in $ B = 6 $
Tetrahedral void occupied $ = 2N $
So, $ N = 6 $
Tetrahedral void= $ 2 \times 6 = 12 $
Now, the number of atoms in A $ = \dfrac{1}{3} \times {\text{Tetrahedral void}} $
$ = \dfrac{1}{3} \times 12 $
$ = 4 $
So, the compound will be $ {A_4}{B_6} $ which can be simplified and written as $ {A_2}{B_3} $
Therefore, option B is the correct answer.
Note :
The closest packing of spheres in two dimensions has a hexagonal symmetry in which every sphere has six nearest neighbors. In hcp, each atom has twelve nearest neighbours. Consider an ideal structure, then the distance between the planes is $ 1.633a $ , where $ a $ is the distance between the atoms of the unit cell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




