Chemical Kinetics Important Questions for NEET Preparation
Chemical Kinetics is a very important chapter of Chemistry that teaches students about various chemical reactions and the factors as well as the mechanism of the reactions. The chapter is in close relation to the physical and chemical processes of reactions that take place. Chemical Kinetics important questions have been offered by the experts of Vedantu to help students understand the chapter and prepare for their NEET examination.
Download Chemical Kinetics NEET questions from Vedantu now and start your preparation early for the medical entrance test. Students will achieve a lot of help from these important questions that have been prepared by the experts at Vedantu. Solving these questions will enable students to gain a thorough knowledge of the chapter.
Access NEET Important Questions Chemistry Chemical Kinetics
1. Rate of reaction of zero first, second-order reaction having the same magnitude of rate constants are ${\gamma _1},{\gamma _2},{\gamma _3}$, respectively Relate ${\gamma _1},{\gamma _2},{\gamma _3}$, if the concentration of reaction is unity.
a. ${\gamma _1} > {\gamma _2} > {\gamma _3}$
b. ${\gamma _1} < {\gamma _2} < {\gamma _3}$
c. ${\gamma _1} = {\gamma _2} = {\gamma _3}$
d. Cannot be predicted.
2. Rate of reaction of zero first, second-order reaction having the same magnitude of rate constants are ${\gamma _1},{\gamma _2},{\gamma _3}$, respectively Relate ${\gamma _1},{\gamma _2},{\gamma _3}$, if the concentration of reaction is lesser than unity.
a. ${\gamma _1} > {\gamma _2} > {\gamma _3}$
b. ${\gamma _1} < {\gamma _2} < {\gamma _3}$
c. ${\gamma _1} = {\gamma _2} = {\gamma _3}$
d. Cannot be predicted.
3. Which of the following represents the fraction of activated molecules in a chemical reaction.
a. $- \dfrac{{{E_\alpha }}}{{RT}}$
b. ${e^{ - {E_a}/RT}}$
c. ${10^{ - {E_a}/RT}}$
d. ${e^{{E_a}/RT}}$
4. For the reaction $P \to Q + R,$ if the initial concentration of x was reduced from 2M to 1M in 20 mins and from 1M to 0.25 M in 40 mins, find the order.
a.1
b.2
c.3
d.0
5. The vant’ Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is [NEET - 2016]
a. 0
b. 1
c. 2
d. 3
6. Which of the following is an example of a consecutive reaction
a. Oxidation of ethane
b. Decomposition of limestone in a closed vessel
c. Nitration of phenol with dilute $HN{O_3}$
d. Oxidation of phenol
7. Half-life of $^{210}Pb$ is 24years. 4 grams of lead is allowed to decay for 12 years, How many leads are left and what is the percentage of decay.
a. Lead left undecayed is 1.414g
b. Lead left undecayed is 2.828g
c. percentage of lead decay is 30%
d. percentage of lead decay is 43%
8. P and Q are two radioactive substances with half-lives 40mins and 50 mins respectively, starting from the equal number of moles of P and Q, after 1 hr, what is the ratio of moles.
a. Ratio of mole left is 1:2
b. Fraction of P left after $5{t_{1/2}}$ is 1/32
c. Fraction of Q left after $3{t_{1/2}}$is 1/20
d. Ratio of mole left is 1:4
9. P and Q are two radioactive substances with half-lives 20mins and 30 mins respectively, starting from the equal number of moles of P and Q, after 1hr, what is the ratio of activity of P and Q.
a. Ratio of mole left is 3:4
b. Ratio of mole left is 1:2
c. Ratio of activities is 3:4
d. Ratio of activities is 1:4
10. The rate of the reaction is given as rate = $k{\left[ X \right]^{3/2}}{\left[ Y \right]^{ - 1/2}}$ rate equation if Y is taken in large excess.
a. Overall order of reaction is 1
b. Overall order of reaction is 2
c. rate= $k{\left[ X \right]^{3/2}}$
d. rate= $k{\left[ Y \right]^{ - 1/2}}$
11. Rate expression for two reactions are:
$rat{e_p} = {k_p}\left[ X \right]$and $rat{e_q} = {k_q}{\left[ Y \right]^2}$. When [X]=[Y]=$1mol{L^{ - 1}}$, ${k_p} = {k_q}mol{L^{ - 1}}$. If [X]=[Y]= $2mol{L^{ - 1}}$, the relation between them ${\text{rat}}{{\text{e}}_{\text{p}}}{\text{ and rat}}{{\text{e}}_{\text{q}}}$.
a. Ratio of rate =1/2
b. Ratio of rates=3/5
c. $\dfrac{{Rat{e_p}}}{{Rat{e_q}}} = \dfrac{{{k_p}[2mol{L^{ - 1}}]}}{{{k_q}{{[2mol{L^{ - 1}}]}^2}}}$
d. $\dfrac{{Rat{e_q}}}{{Rat{e_p}}} = \dfrac{{{k_q}[2mol{L^{ - 1}}]}}{{{k_p}{{[2mol{L^{ - 1}}]}^2}}}$
12. In a bimolecular reaction, the steric factor P was experimentally determined to be 4.5. The correct option(s) among the following is(are)
a. The activation energy of the reaction is unaffected by the value of the steric factor
b. the Experimentally determined value of frequency factor is higher than that predicted by Arrhenius equation
c. Since P = 4.5, the reaction will not proceed unless an effective catalyst is used
d. The value of the frequency factor predicted by the Arrhenius equation is higher than that determined experimentally
13. According to the Arrhenius equation
a. High activation energy usually means a fast reaction
b. The rate constant increases with increasing temperature. This is because the number of collisions is high and the energy exceeds the activation energy.
c. The higher the activation energy, the stronger the temperature dependence of the rate constant.
d. The pre-exponential factor is a measure of the velocity at which a collision occurs, regardless of energy.
14. In the decay sequence, ${ }_{92}^{238} \mathrm{U} \stackrel{-\mathrm{x}_{1}}{\longrightarrow}{ }_{90}^{234} \mathrm{Th} \stackrel{-\mathrm{x}_{2}}{\longrightarrow}{ }_{91}^{234} \mathrm{~Pa} \stackrel{-\mathrm{x}_{3}}{\longrightarrow}{ }^{234} \mathrm{Z} \stackrel{-\mathrm{x}_{4}}{\longrightarrow}{ }_{90}^{230} \mathrm{Th}$
${x_1}$, ${x_2}$, ${x_3}$ and ${x_4}$ are particles/radiation emitted by the respective isotopes. The correct option(s) is (are)
A. ${x_3}$is γ-ray
B. Z is an isotope of uranium
C. ${x_1}$ will deflect towards the negatively charged plate
D. ${x_2}$ is β–
15. ${N_2} + 3{H_2} \to 2N{H_{3.}}$the rate of disappearance of nitrogen is $0.002mol{L^{ - 1}}{S^{ - 1}}$. What is the rate of appearance of ammonia?
A. $0.002mol{L^{ - 1}}{S^{ - 1}}$
B. $0.004mol{L^{ - 1}}{S^{ - 1}}$
C. $0.68g{L^{ - 1}}{S^{ - 1}}$
D. $0.02g{L^{ - 1}}{S^{ - 1}}$
16. ${P_2}Q$ is an ideal gas, which decomposes according to the equation: ${P_2}Q \to {P_2} + \dfrac{1}{2}{Q_2}$. At the start, the initial pressure is 100 mm Hg and after 5 mins, the pressure is 120 mm Hg What is the average rate of decomposition od ${P_2}Q$,
A. 0.133 mm s-1
B. 8 mm min-1
C. a decrease in pressure is 40mm
D. T and V are constant
17. For the following reaction $2X + Y\xrightarrow{K}P$ the rate of reaction is $\dfrac{{d\left[ P \right]}}{{dt}} = k\left[ X \right]$ Two moles of X are mixed with one mole of Y to make 1.0L of solution. At 50s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are) (ADV-2021)
A. The rate constant, k of the reaction is $13.86 \times {10^{ - 4}}{s^{ - 1}}$
B. Half-life of X is 50 s.
C. At 50s, $ - \dfrac{{d[X]}}{{dt}} = 13.86 \times {10^{ - 3}}mol{L^{ - 1}}{s^{ - 1}}$
D. At 100 s, $ - \dfrac{{d[Y]}}{{dt}} = 3.46 \times {10^{ - 3}}mol{L^{ - 1}}{s^{ - 1}}$
18. Consider the following figure and mark the correct option. (NEET 2011)
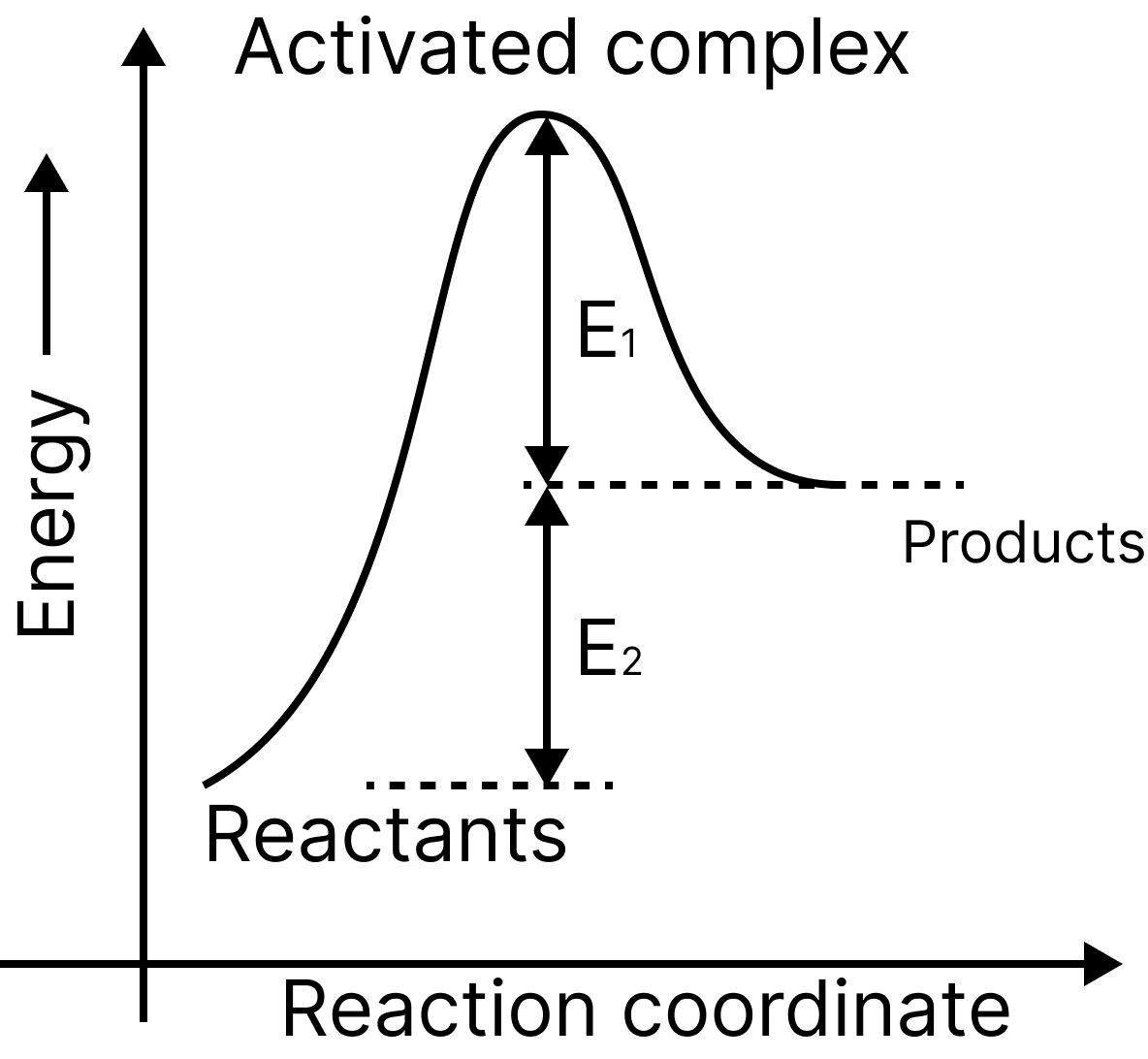
(a) Activation energy of forwarding reaction is E1 + E2 and the product is less stable than the reactant.
(b) Activation energy of forwarding reaction is E1 + E2 and the product is more stable than the reactant.
(c) Activation energy of both forward and backward reactions is E1 + E2 and reactant is more stable than a product.
(d) Activation energy of the backward reaction is E, and the product is more stable than the reactant.
19. The rate, at which a substance reacts, depends upon its (AIIMS-1996)
A. equivalent mass
B. Molecular mass
C. Active mass
D. Atomic mass.
20. When a biochemical reaction is carried out in a laboratory from outside of the human body in the absence of an enzyme then of reaction Obtained is 10−6 times, then the activation energy of a reaction in the presence of an enzyme is: (AIPMT 2001)
A. $\dfrac{6}{{RT}}$
B. P is required
C. Different from Ea. obtained in laboratory
D. Cannot say anything
Assertion and Reason (R) type questions.
1) Both (A) and (R) are true and (R) is the correct explanation of (A)
2) Both (A) and (R) are true and (R) is not the correct explanation of (A)
3) (A) is true but (R) is false
4) Both (A) and (R) are false.
21. (A): Spontaneous reaction may be slow or fast
(R): Spontaneous nature deals with the feasibility of the reaction but not the rate.
22. (A): Lesser the activation energy, the greater the rate of reaction
(R): Activation energy of a reaction is independent of temperature.
23. (A): The rate of the reaction is the rate of change of concentration of a reactant or a product
(R): Rate of reaction remains constant during the complete reaction.
24. (A): The overall order of the reaction is the sum of the exponents of all the reactants in the rate expression.
(R): There are many higher-order reactions. (NEET 2010)
25. (A): Catalyst changes Gibbs's free energy of the system.
(R): Catalyst changes the pre-exponential factor of a chemical reaction. (NEET 2013)
Solutions:
1. C
Suppose ${\gamma _1},{\gamma _2},{\gamma _3}$are the rate of three reactions of the first second, and third-order, and k is the rate constant all the three reactions will be
${\gamma _1} = K[A]$
${\gamma _2} = K{[A]^2}$
${\gamma _3} = K{[A]^3}$
[A] being the concentration of the reaction A in mole per lit, then
For unity[A] =${\gamma _1} = {\gamma _2} = {\gamma _3}$
2. A
Suppose ${\gamma _1},{\gamma _2},{\gamma _3}$are the rate of three reactions of first second, and third-order, and k is the rate constant all the three reactions will be
${\gamma _1} = K[A]$
${\gamma _2} = K{[A]^2}$
${\gamma _3} = K{[A]^3}$
[A] being the concentration of the reaction A in mole per lit, then
For A lesser than 1 =${\gamma _1} > {\gamma _2} > {\gamma _3}$
3. B
According to the Arrhenius equation, $K = A{e^{ - {E_a}/RT}}$ where, ${e^{ - {E_a}/RT}}$ represents a fraction of molecule having energy equal to or greater than activation energy, Ea.
4. A
Half-life, ${\text{(}}{{\text{t}}_{{\text{1/2}}}}{\text{) \alpha }}{{\text{a}}^{{\text{1 - n}}}}$
Here, half-life is independent of the initial concentration, and the order of the reaction is 1
5. B
Note: For strong electrolytes, van't Hoff factor (i) is equal to the number of ions.
$\mathrm{Ba}(\mathrm{OH})_{2} \rightarrow \mathrm{Ba}^{2+}+2 \mathrm{OH}^{+}$
The number of ions is equal to $(1+2=3)$
Use the van't Hoff factor (i) formula
$\alpha=\frac{i-1}{n-1}$
$1=\frac{i-1}{3-1}$
$\mathrm{i}=2+1$
$\mathrm{i}=3$
6. A, B, C
The reactions in which the reactant forms an intermediate and the intermediate forms the product in one or many subsequent reactions are called consecutive reactions
7. B, D
Radioactive decay follows the first-order kinetic number of half-lives
Number of half-lives
${\text{(n) = }}\dfrac{{{\text{total time}}}}{{{\text{Half - life}}}}{\text{ = }}\dfrac{{{\text{12y}}}}{{{\text{24y}}}}{\text{ = 0}}{\text{.5}}$
Amount of lead left undecayed
$\dfrac{{{\text{Amount of lead taken}}}}{{{{\text{2}}^{\text{n}}}}}{\text{ = }}\dfrac{{\text{4}}}{{\sqrt {\text{2}} }}{\text{ = 2}}{\text{.828g}}$
Amount of lead decayed = amount taken – left amount
= 4-2.828 = 1.172 g
Percentage of the lead decay is $\dfrac{{1.172 \times 100}}{4} = 43.00\% $
8. a, b
In the given time, 1 hr is $5{t_{1/2}}$ for the substance P and $4{t_{1/2}}$for the substance Q
The fraction of p left after $5{t_{1/2}}$ = $\dfrac{1}{{{2^5}}} = \dfrac{1}{{32}}$
The fraction of p le16ft after $4{t_{1/2}}$ = $\dfrac{1}{{{2^4}}} = \dfrac{1}{{16}}$
Ration of moles left = $\dfrac{1}{{32}}:\dfrac{1}{{16}}$=1:2
9. b, c
In the given time, 1 hr is $3{t_{1/2}}$ for the substance P and $2{t_{1/2}}$for the substance Q
The fraction of p left after $3{t_{1/2}}$ = $\dfrac{1}{{{2^3}}} = \dfrac{1}{8}$
The fraction of p le16ft after $2{t_{1/2}}$ = $\dfrac{1}{{{2^2}}} = \dfrac{1}{4}$
Ration of moles left = $\dfrac{1}{8}:\dfrac{1}{4}$=1:2
The ratio of activity
$\dfrac{{{\lambda _P}{N_P}}}{{{\lambda _Q}{N_Q}}} = \dfrac{{{N_P}{{\left( {{t_{1/2}}} \right)}_P}}}{{{N_Q}{{\left( {{t_{1/2}}} \right)}_Q}}} = \dfrac{{\left( {1/8} \right) \times 30}}{{\left( {1/4} \right) \times 20}} = 3:4$
10. a c
The rate law of the reaction is given as rate = $k{\left[ X \right]^{3/2}}{\left[ Y \right]^{ - 1/2}}$
Order with respect to X is $\dfrac{3}{2}$
Order with respect to Y is $\dfrac{-1}{2}$
The overall order of the reaction is $\dfrac{3}{2}-(\dfrac{-1}{2})=1$
If y is taken largely in excess the rate is independent of its concentration, as [Y] is taken as constant during the course of the reaction.
The rate equation when X is isolated is rate= $k{\left[ X \right]^{3/2}}$
11. a, c
$rat{e_p} = {k_p}\left[ {2mol{L^{ - 1}}} \right]$
$rat{e_q} = {k_q}{\left[ {2mol{L^{ - 1}}} \right]^2} = {k_p}{\left[ {2mol{L^{ - 1}}} \right]^2}$.
The ratio of the rate of two reactions (p) and (q) is $\dfrac{{Rat{e_p}}}{{Rat{e_q}}} = \dfrac{{{k_p}[2mol{L^{ - 1}}]}}{{{k_q}{{[2mol{L^{ - 1}}]}^2}}} = \dfrac{1}{2}$
The ratio of the rates =1: 2
12. A, B
ρ = 4.5 ∴ ρ > 1
We know, $K_{e x p} / K_{\text {collison }}=\rho$
$K_{\text {exp }}=\rho A e^{-E a} / R T$
$A_{e x p}=\rho A$
$\therefore A_{e x p}>A$
The experimentally determined value of the frequency factor is higher than that predicted by the Arrhenius equation.
13. B, C, D
According to Arrhenius's Equation
$K{\text{ }} = {\text{ }}A{e^{ - Ea}}/RT$
Therefore, at T → 0
${e^{ - Ea}}/RT \rightarrow 0$
Therefore, rate constant decreases with temperature.
And as T → ∞ K → A.
Thus, as temperature increases, the rate constant increases and approaches a value close to the Arrhenius constant.
Now
$dK/dT = - EaA/RT{\text{ }}{e^{ - Ea}}/RT$
Hence, the Higher the activation energy higher is the required temperature for reaction to occur, and hence greater the temperature dependency of the rate constant.
14. B, C, D
${ }_{92} \mathrm{U}^{238} \longrightarrow{ }_{90} \mathrm{Th}^{234}+{ }_{2} \mathrm{He}^{4}$
${ }_{90} \mathrm{Th}^{234} \longrightarrow{ }_{91} \mathrm{~Pa}^{234}+{ }_{-1} \mathrm{e}^{0}$
${ }_{91} \mathrm{~Pa}^{234} \longrightarrow{ }_{92} \mathrm{Z}^{234}+{ }_{-1} \mathrm{e}^{0}$
${ }_{92} \mathrm{Z}^{234} \longrightarrow{ }_{90} \mathrm{Th}^{230}+{ }_{2} \mathrm{He}^{4}$
$\therefore \quad x_{1}=\alpha ; x_{2}=x_{3}={ }_{-1} \beta^{0} ; x_{4}=\alpha$
15. B, C
$\dfrac{{\Delta [{N_2}]}}{{\Delta t}} = 0.02mol{L^{ - 1}}{s^{ - 1}}$
$= \dfrac{1}{2}\dfrac{{\Delta [N{H_3}]}}{{\Delta t}}$
The rate of appearance of ammonia is $0.004mol{L^{ - 1}}{S^{ - 1}}$ it is also given as $0.68g{L^{ - 1}}{S^{ - 1}}$
16. A,B,C,D
The decomposition reaction of gaseous ${P_2}Q$ is given as
${P_2}Q \to {P_2} + \dfrac{1}{2}{Q_2}$
T and V are constant
At the start of the reaction : 100 0 0
After the reaction: 100-2x 2x x
100-2x+2x+x=100+x=120mm
X=20mm or 2x= 40mm
The decrease in pressure of reactant substance, ${P_2}Q$ in 5mins, is 40 mm
The rate decomposition of ${P_2}Q$=$\dfrac{{{\text{40}}}}{{\text{5}}}{\text{ = 8mm mi}}{{\text{n}}^{{\text{ - 1}}}}{\text{ = 0}}{\text{.133mm }}{{\text{s}}^{{\text{ - 1}}}}$
17. B, C, D
$\begin{array}{lllll}& 2 X & y & & \\t=0 & 2 & 1 & & \\ t=50 & 2-2 \times 0.5 & 1-0.5 & & \\& \text { lmole } & 0.5 &\end{array}$
$rate = - \dfrac{1}{2}\dfrac{{d[X]}}{{dt}} = - \dfrac{{d[Y]}}{{dt}} = k[X]$
$- \dfrac{{d[X]}}{{dt}} = 2k[X] = k[X]$
Half-life t=50s
$2k = \dfrac{{0.693L}}{{50}}$
Rate constant, (k)=$\dfrac{{0.6932}}{{100}} = 6.932 \times {10^{ - 3}}$
${t_{\dfrac{1}{2}}} = 50s$
$- \dfrac{{d[X]}}{{dt}} = 2k[X]$
At 50 s $- \dfrac{{d[X]}}{{dt}} = 2 \times 6.932 \times {10^{ - 03}} \times 1$
$= 13.86 \times {10^{ - 3}}mol{L^{ - 1}}{s^{ - 1}}$
At 50s, $- \dfrac{{d[X]}}{{dt}} = 13.86 \times {10^{ - 3}}mol{L^{ - 1}}{s^{ - 1}}$
At 100 s, $- \dfrac{{d[Y]}}{{dt}} = 3.46 \times {10^{ - 3}}mol{L^{ - 1}}{s^{ - 1}}$
18. A
Ea. (forward) = E1 + E2
Since the energy of reactants is less than products and the product is less stable than the reactant.
19. C
The rate, at which a substance reacts, depends upon its active mass.
20. C
When a biochemical reaction is carried out in the laboratory from outside of the human body in the absence of an enzyme, the rate of reaction obtained is 10−6 times the activation energy of a reaction in the presence of the enzyme. It is different from Ea. obtained in the laboratory because for a given chemical reaction $K = A{e^{ - Ea}}/RT$ (Arrhenius equation)
21. A
The spontaneous reaction may be slow or fast.
Spontaneous nature deals with the feasibility of the reaction but not the rate.
22. B
The lesser the activation energy, the greater the rate of reaction
The activation energy of a reaction is independent of temperature is not true as ${E_a} = 0$
T is not dependent on K
23. C
The rate of reaction does not remain constant during the complete reaction because the rate depends upon the concentration of reactants which decreases with time.
24. C
Reactions of higher order are rare because chances for a larger number of molecules to come simultaneously for collision are less.
25. D
Catalyst has no effects on Gibb's free energy of the system and the pre-exponential factor of a chemical reaction
Importance of Physical Chemistry Chemical Kinetics
Students can learn a lot about chemical reactions from this chapter. For candidates who are preparing for NEET, having a sound grasp of different concepts of Chemistry is really important. Since chemical reactions are an essential part of the subject, students can take some help from the Chemical Kinetics important questions PDF to prepare the chapter in an efficient manner. Students are introduced to different concepts and subtopics related to chemical kinetics in the chapter such as rate of concentration, dependence on the rate of concentration, collision theory, catalysts, molecularity mechanism, temperature dependence, integrated equations, and a lot more.
The chapter details all the important factors that have a role to play in the chemical reactions that take place. After careful study of the chapter, students will learn about the Half-Life of any reaction which is the time taken by the reactant’s concentration to reduce to a half of what it was earlier. Students can go through different concepts such as Zero Order reactions, First Order Reactions, as well as practical methods to find out the order of any given reaction.
Chemical Kinetics is a fundamental part of Physical Chemistry and by solving different questions and answering the problems of the chapter, students will get enough help and confidence to perform well in their NEET entrance test. These questions have detailed answers that can help the students balance equations, perform practical tests, and answer long-answer questions from the chapter. Downloading the Chemical Kinetics Class 12 important questions would surely be a good idea.
Benefits of Vedantu’s Chemical Kinetics Important Questions
Students can develop a sound understanding of the chapter by solving the questions that have been prepared by the subject matter experts at Vedantu. They can understand the concepts of chemical reactions and their processes easily. They can firmly grasp the details of different terms such as Half-Life, temperature dependence, molecularity, and much more.
Chemical Kinetics important questions and answers have been created by Vedantu experts who are well-versed in the concepts of the NEET syllabus. So, the solutions to the questions are going to be accurate and reliable.
If there are any sections where the students need some help or clarification, these questions and answers seem to be very helpful. They can cross-check their answers with the ones provided by Vedantu and rectify any mistakes that they might have made.
Download Important Questions for Chemical Kinetics from Here
If you are interested in scoring high marks in your upcoming NEET exam and need some help with Chemical Kinetics, download the PDF files of Chemical Kinetics important questions for NEET. These questions are the right study materials that you need to nail the exam and get a high rank in the results. Learn all the details about chemical kinetics and practice from these given sets of questions to revise the chapter in the best way.
Other Important Links
S. No | Other Important Links for NEET Transport in Plants |
|---|---|
1 | |
2 |
NEET Chemistry Important Questions - Chapter Pages
NEET Chemistry Chapter-wise Important Questions | |
FAQs on Chemical Kinetics Class 12 Important Questions NEET Chemistry (Free PDF Download)
1. When can the order of a reaction become zero?
The order of any reaction is a term used to express the sensitivity level of the reaction to any particular change taking place in the concentration of the reactants. This level can be zero when there isn’t any change in the reaction rate even after an alteration has been made to the reactants’ concentrations.
2. What does the term ‘rate of a reaction’ mean?
The rate of a reaction can be defined as the concentration change in any of the products or reactants in a given period of time. Also, the rate of change in molar concentration of any species that takes place in a reaction is also called the rate of a reaction.
3. Define molecularity and state its classification.
Molecularity can be defined as the number of reaction species (molecules, ions, and atoms) that take part in any elementary reaction that has to collide and result in a chemical reaction. Molecularity can be classified into Unimolecular, Bimolecular, and Trimolecular reactions.
4. What is activation energy?
As per the Collision Theory, any reaction is a result of the collision of reactant molecules with each other. The minimum additional energy absorbed by the reactant molecules for the energy levels to be equal to the threshold energy is known as the activation energy.

























