
Which one is correct about the given P-V plot for 2 mole of an ideal gas?
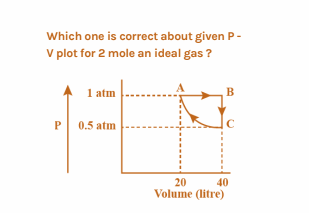
A. ${W_{AB}} = - 20L - atm$
B. $\Delta u$ for cycle = 0
C. $\Delta {S_{cycle}}$ = 0
D. ${W_{CA}} = 13.86 Latm$
Answer
233.1k+ views
Hint: For an ideal gas, from the ideal gas law, P-V remains constant through an isothermal process. For an isothermal, reversible process, the work done by the gas is equal to the area under the relevant pressure-volume isotherm.
Formula used:
${W_{AB}} = - {P_{ext}}({V_2} - {V_1})$
$\Delta S + nR\ln (\dfrac{{{v_2}}}{{{v_1}}}) + n{C_V}\ln (\dfrac{{{T_2}}}{{{T_1}}})$
Complete step by step answer:
Now,
According to formula A,
${W_{AB}} = - {P_{ext}}({V_2} - {V_1})$
$ = - 1(40 - 20)$
$ = - 20L $atm
$\Delta u$ For cycle =0 (as initial and final temperatures are same)
$\Delta S = 0$ As initial and final states are same (according to formula B )
Now, the work done in AC=Work done in ABCA-Work done in AB
$ = \dfrac{{\pi \times 0.5 \times 20}}{4} - 20$
$ = - 12.15L atm$
Hence, we can see that option A, B, C are correct.
Note:
There is no “real” gas that is truly an ideal gas. An ideal gas is a theoretical concept which has single points moving randomly with perfectly elastic collisions. All molecules have a volume and intermolecular forces of attraction. So a real molar volume is different from an ideal molar volume.
Formula used:
${W_{AB}} = - {P_{ext}}({V_2} - {V_1})$
$\Delta S + nR\ln (\dfrac{{{v_2}}}{{{v_1}}}) + n{C_V}\ln (\dfrac{{{T_2}}}{{{T_1}}})$
Complete step by step answer:
Now,
According to formula A,
${W_{AB}} = - {P_{ext}}({V_2} - {V_1})$
$ = - 1(40 - 20)$
$ = - 20L $atm
$\Delta u$ For cycle =0 (as initial and final temperatures are same)
$\Delta S = 0$ As initial and final states are same (according to formula B )
Now, the work done in AC=Work done in ABCA-Work done in AB
$ = \dfrac{{\pi \times 0.5 \times 20}}{4} - 20$
$ = - 12.15L atm$
Hence, we can see that option A, B, C are correct.
Note:
There is no “real” gas that is truly an ideal gas. An ideal gas is a theoretical concept which has single points moving randomly with perfectly elastic collisions. All molecules have a volume and intermolecular forces of attraction. So a real molar volume is different from an ideal molar volume.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




