
Which of the following options gives ethyl benzene with phenyl methyl ketone?
A. \[Zn - Hg + HCl\]
B. \[LiAl{H_4}\]
C. \[KMn{O_4}\]
D. None of the above
Answer
233.1k+ views
Hint: Phenyl Methyl ketone (Acetophenone) is the organic compound with the formula \[{C_6}{H_5}CC{H_3}\]. It is known as the simplest aromatic ketone. It is a colourless and viscous liquid and is useful in making resins and fragrances as a precursor. The ketone group of the Phenyl methyl ketone compound is being reduced in this reaction.
Complete Step by Step Solution:
For reducing aldehydes or ketones, zinc amalgam along with HCl is used. This reaction which reduces ketones or aldehydes is also known as Clemmensen's reduction reaction. It is named after the famous Danish chemist, Erik Christian Clemmensen. The reaction is carried out below:
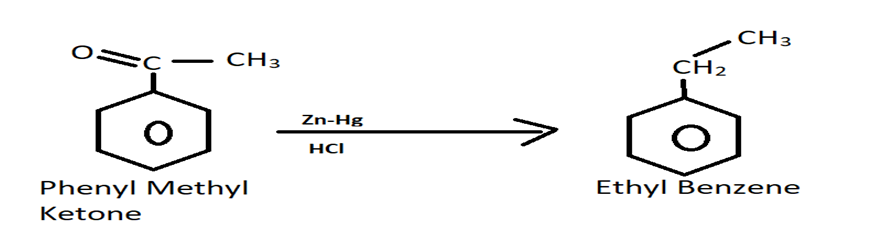
Image: Chemical Reaction
Thus, the correct answer to this question will be, option (A).
Additional Information:
Alkane from alkenyl chloride (halide) can be prepared from any organic compound which is transformed into alkenyl halide. This reaction is widely used to convert the carbonyl group into a methyl group. Preparation of polycyclic aromatics and aromatics containing the unbranched sides of the hydrocarbon chain. The reaction helps to reduce the aliphatic and the mixed aliphatic-aromatic carbonyl compounds. The Clemmensen reduction is most widely used to transform acyl benzene (from acylation by Friedel-Crafts) to alkylbenzene.
Note: The mechanism of the Clemmensen reduction reaction is not well known. Still, there are two proposals for its mechanism:
1) Carbanionic Mechanism: In the Carbanionic mechanism, zinc attacks directly the protonated carbon.
2) Carbenoid Mechanism: It's a radical process that reduces the happenings on the metal surface of zinc. The reaction of the carbenoid mechanism always takes place at the surface of the zinc catalyst.
Complete Step by Step Solution:
For reducing aldehydes or ketones, zinc amalgam along with HCl is used. This reaction which reduces ketones or aldehydes is also known as Clemmensen's reduction reaction. It is named after the famous Danish chemist, Erik Christian Clemmensen. The reaction is carried out below:

Image: Chemical Reaction
Thus, the correct answer to this question will be, option (A).
Additional Information:
Alkane from alkenyl chloride (halide) can be prepared from any organic compound which is transformed into alkenyl halide. This reaction is widely used to convert the carbonyl group into a methyl group. Preparation of polycyclic aromatics and aromatics containing the unbranched sides of the hydrocarbon chain. The reaction helps to reduce the aliphatic and the mixed aliphatic-aromatic carbonyl compounds. The Clemmensen reduction is most widely used to transform acyl benzene (from acylation by Friedel-Crafts) to alkylbenzene.
Note: The mechanism of the Clemmensen reduction reaction is not well known. Still, there are two proposals for its mechanism:
1) Carbanionic Mechanism: In the Carbanionic mechanism, zinc attacks directly the protonated carbon.
2) Carbenoid Mechanism: It's a radical process that reduces the happenings on the metal surface of zinc. The reaction of the carbenoid mechanism always takes place at the surface of the zinc catalyst.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




