
Which of the following is (are) true regarding intermediate in the addition-elimination mechanism of the reaction given?
I. The intermediate is aromatic.
II. The intermediate is resonance stabilized anion.
III. Electron withdrawing group on the benzene ring stabilizes the intermediate.
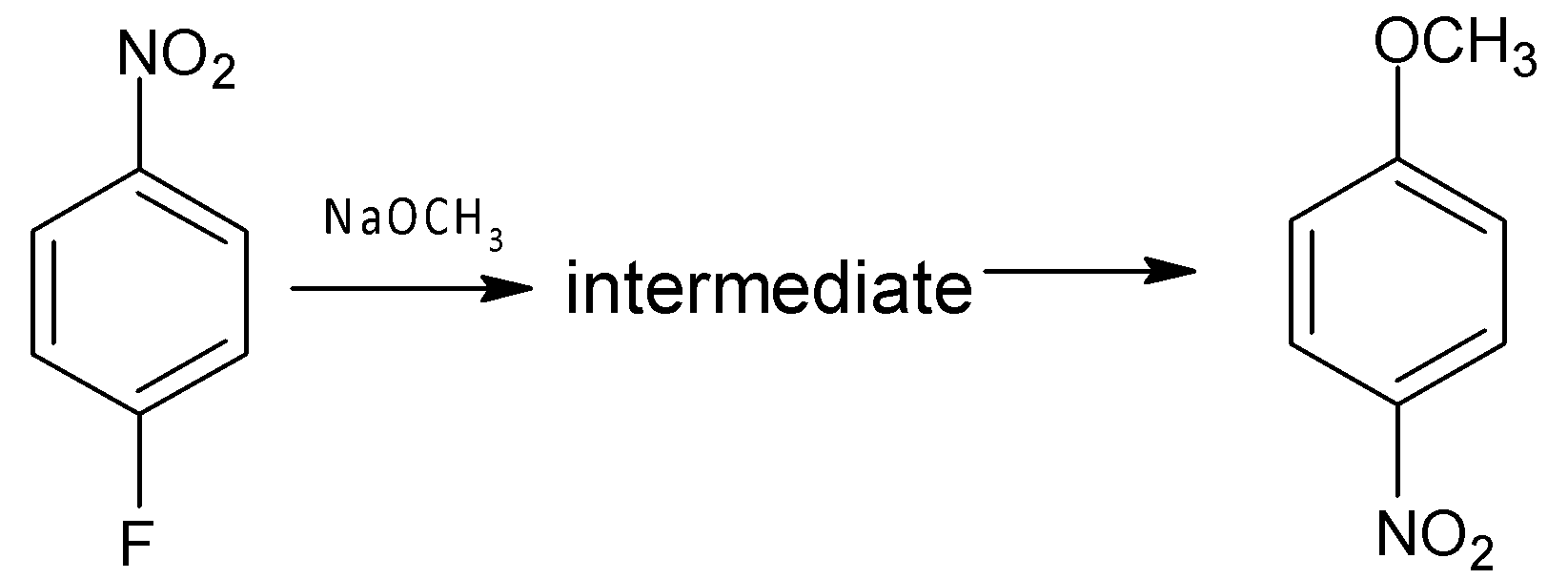
A. I only
B. II only
C. I and III
D. II and III
Answer
232.8k+ views
Hint: When an electron-deficient aromatic ring which consists of a leaving group undergoes substitution with electron-rich nucleophiles. This nucleophilic aromatic substitution reaction also known as the addition-elimination reaction proceeds via an electron-rich Meisenheimer intermediate.
Complete step by step solution:
The overall mechanism of addition-elimination reaction in nitro-substituted aryl halides occurs by a two-step mechanism. In the first step, nucleophile ${{(}^{-}}OC{{H}_{3}})$ attacks nitro-substituted aromatic ring, forming an anionic intermediate named very popularly Meisenheimer intermediate. This is the step of addition, where aromaticity of ring losses. The ring restores its aromaticity by removing or leaving group F at a very fast rate in the next elimination step. Here a strong electron withdrawing group affects the rate of addition-elimination reaction, as the presence of an electron-withdrawing group (here nitro group in para position) stabilizes the intermediate (cyclohexadienyl anion) by resonance.
Mechanism:
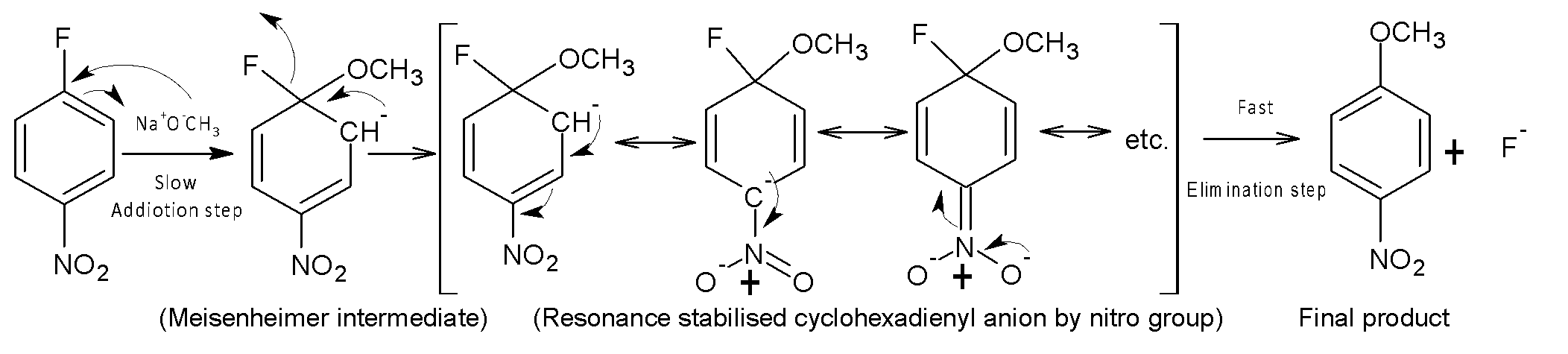
The name of the intermediate formed in the reaction is cyclohexadiene anion is not an aromatic compound. The presence of EWG in para position (p-$N{{O}_{2}}$) on the benzene ring stabilizes the intermediate by resonance. The higher the number of electron-withdrawing groups in the ring at the ortho, para position, or both, the higher will be the rate of nucleophilic aromatic substitution reaction.
Therefore, (I) is not correct, and (II) and (III) is appropriate for the intermediate formed in the addition-elimination reaction.
Thus, Option (D) is correct.
Note: Aromatic nucleophilic substitution reaction affects the leaving group. Generally, fluorine (F) is used due to its very high electronegativity. The reactivity of order of aryl halides: -F>-Cl>-Br>-I. The overall reaction is of second order (addition+emanation).
Complete step by step solution:
The overall mechanism of addition-elimination reaction in nitro-substituted aryl halides occurs by a two-step mechanism. In the first step, nucleophile ${{(}^{-}}OC{{H}_{3}})$ attacks nitro-substituted aromatic ring, forming an anionic intermediate named very popularly Meisenheimer intermediate. This is the step of addition, where aromaticity of ring losses. The ring restores its aromaticity by removing or leaving group F at a very fast rate in the next elimination step. Here a strong electron withdrawing group affects the rate of addition-elimination reaction, as the presence of an electron-withdrawing group (here nitro group in para position) stabilizes the intermediate (cyclohexadienyl anion) by resonance.
Mechanism:

The name of the intermediate formed in the reaction is cyclohexadiene anion is not an aromatic compound. The presence of EWG in para position (p-$N{{O}_{2}}$) on the benzene ring stabilizes the intermediate by resonance. The higher the number of electron-withdrawing groups in the ring at the ortho, para position, or both, the higher will be the rate of nucleophilic aromatic substitution reaction.
Therefore, (I) is not correct, and (II) and (III) is appropriate for the intermediate formed in the addition-elimination reaction.
Thus, Option (D) is correct.
Note: Aromatic nucleophilic substitution reaction affects the leaving group. Generally, fluorine (F) is used due to its very high electronegativity. The reactivity of order of aryl halides: -F>-Cl>-Br>-I. The overall reaction is of second order (addition+emanation).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




