
Which of the following is allylic halide?
(a) (1-Bromoethyl) benzene
(b) Benzyl chloride
(c) 1-Bromo benzene
(d) 3-Chloro cyclohex-1-ene
Answer
233.1k+ views
Hint: An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
Step-by-step answer:
> In (1-Bromoethyl) benzene or \[{{C}_{8}}{{H}_{9}}Br\], we can see that on the allylic carbon of the molecule, the halogen atom is present with a methyl group. Hence, it cannot be termed as an allylic halide.
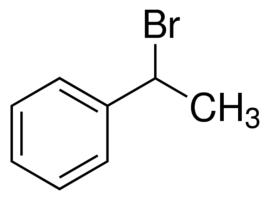
(1-Bromoethyl) benzene
> In Benzyl chloride or (Chloromethyl) Benzene with a molecular formula of \[{{C}_{7}}{{H}_{7}}Cl\], we can see that on the allylic carbon of the molecule, the halogen atom is present along with a methyl group. This is why Benzyl chloride does not qualify as an allylic halide.
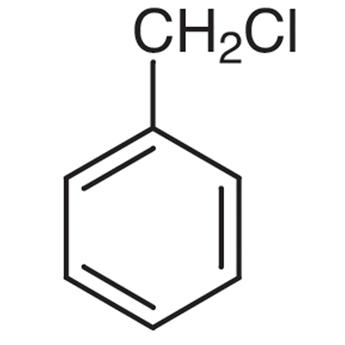
Benzyl chloride
> 1-Bromo benzene or \[{{C}_{6}}{{H}_{5}}Br\] as we can see does not have the halogen atom Br bonded to \[s{{p}^{3}}-hybridised\] carbon atom next to the Carbon-Carbon double bond \[\left( C=C \right)\]. This denotes that 1-Bromo benzene is not an allylic halide.
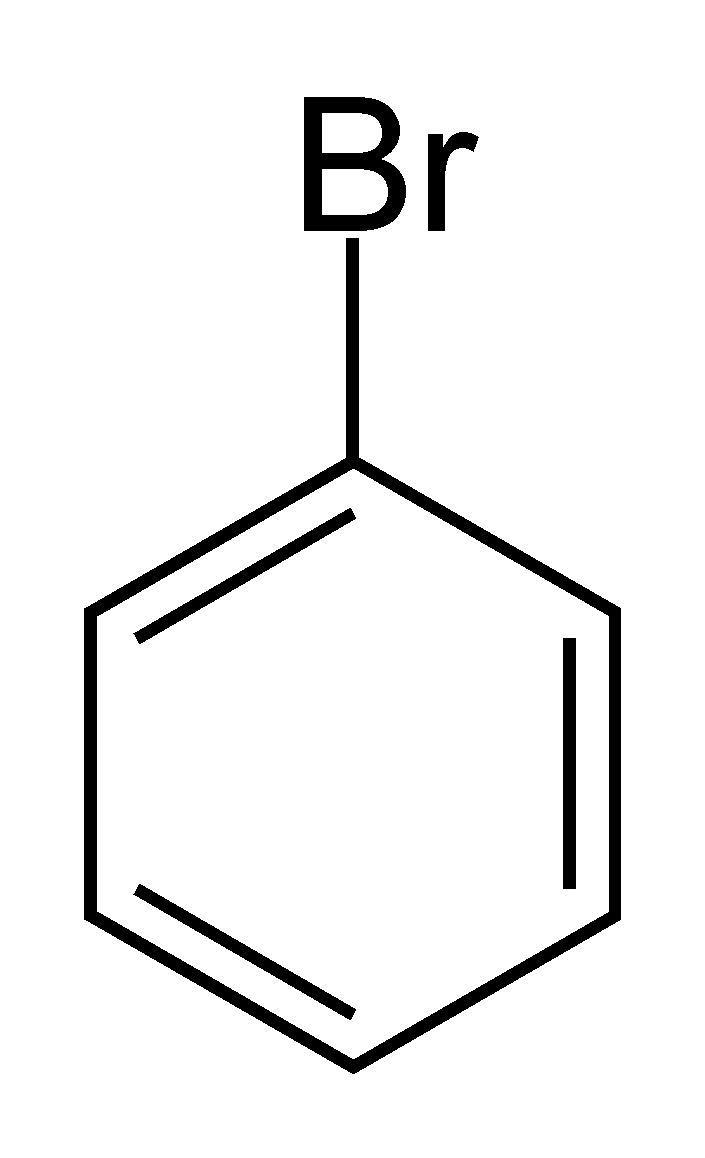
1-Bromo benzene
> An allylic halide is an alkyl halide which has one or more halogen atoms on the allylic carbons of the molecule. In the below given figure of 3-Chloro cyclohex-1-ene, we can see a halogen atom bonded to \[s{{p}^{3}}-hybridised\] carbon atom next to the Carbon- Carbon double bond \[\left( C=C \right)\]. This denotes that 3-Chloro cyclohex-1-ene or \[{{C}_{7}}{{H}_{11}}Cl\] is allylic halide.
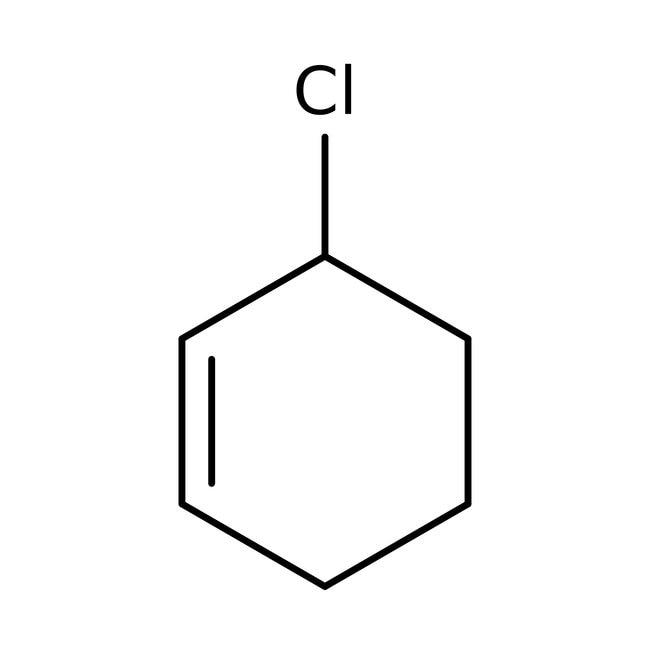
3-Chloro cyclohex-1-ene
So, we can figure out that Option D is the correct answer.
Note: Allylic halide is the one in which the carbon atom next to the double bonded carbon atom carries one or more halogen atoms. Allylic halides are reactive in both \[{{S}_{N}}1\] and \[{{S}_{N}}2\] mechanisms.
Step-by-step answer:
> In (1-Bromoethyl) benzene or \[{{C}_{8}}{{H}_{9}}Br\], we can see that on the allylic carbon of the molecule, the halogen atom is present with a methyl group. Hence, it cannot be termed as an allylic halide.

(1-Bromoethyl) benzene
> In Benzyl chloride or (Chloromethyl) Benzene with a molecular formula of \[{{C}_{7}}{{H}_{7}}Cl\], we can see that on the allylic carbon of the molecule, the halogen atom is present along with a methyl group. This is why Benzyl chloride does not qualify as an allylic halide.

Benzyl chloride
> 1-Bromo benzene or \[{{C}_{6}}{{H}_{5}}Br\] as we can see does not have the halogen atom Br bonded to \[s{{p}^{3}}-hybridised\] carbon atom next to the Carbon-Carbon double bond \[\left( C=C \right)\]. This denotes that 1-Bromo benzene is not an allylic halide.

1-Bromo benzene
> An allylic halide is an alkyl halide which has one or more halogen atoms on the allylic carbons of the molecule. In the below given figure of 3-Chloro cyclohex-1-ene, we can see a halogen atom bonded to \[s{{p}^{3}}-hybridised\] carbon atom next to the Carbon- Carbon double bond \[\left( C=C \right)\]. This denotes that 3-Chloro cyclohex-1-ene or \[{{C}_{7}}{{H}_{11}}Cl\] is allylic halide.

3-Chloro cyclohex-1-ene
So, we can figure out that Option D is the correct answer.
Note: Allylic halide is the one in which the carbon atom next to the double bonded carbon atom carries one or more halogen atoms. Allylic halides are reactive in both \[{{S}_{N}}1\] and \[{{S}_{N}}2\] mechanisms.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




