
Which of the following gives a positive Libermann-nitroso test?
(A) 2-butanamine
(B) N-ethyl-2-pentanamine
(C) N-methylpiperidine
(D) N,N-dimethylcyclohexylamine
Answer
233.1k+ views
Hint: The Libermann-nitroso test which is used for the test of secondary amines that may be aliphatic or aromatic. In the test, the secondary amines react with nitrous acid and formation of N-nitrosamines takes place which are water soluble yellow oils which convert into violet or greenish blue on further treatment with alkali.
Complete Step by Step Solution:
As we know that only secondary amines will give a positive result to the Libermann-nitroso test. So, among the given options we have to compare the structures of the given amines as follows:
Structure (A): 2-butanamine
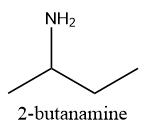
Structure (B): N-ethyl-2-pentanamine
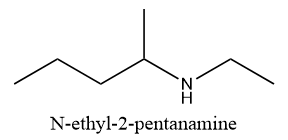
Structure (C): N-methylpiperidine

Structure (D): N,N-dimethylcyclohexylamine
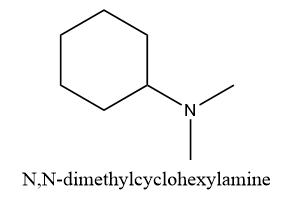
Secondary amines are the amines in which the amino group i.e The nitrogen atom is bonded directly to the two carbon atoms of any hybridization but the carbon atoms must not be carbonyl group carbons.
So, among the given options, structure (A) is eliminated because the nitrogen atom of the amino group is bonded to a single carbon atom only. Structures (C) and (D) are eliminated as well because the nitrogen atom of the amino group in both the compounds are bonded to three carbon atoms i.e., the structure (C) and (D) are the tertiary amines.
In structure B, the nitrogen atom is bonded to two carbon atoms and one hydrogen atom and thus, is a secondary amine which shows positive Liberman-nitroso test. Hence, a positive Libermann-nitroso test will be given by N-ethyl-2-pentanamine.
Therefore, option (B) is the correct answer.
Note: It is important to note that the Libermann-nitroso test is also employed for the test of phenol as on reaction, it forms a deep green or blue colour solution which converts to red on dilution with water.
Complete Step by Step Solution:
As we know that only secondary amines will give a positive result to the Libermann-nitroso test. So, among the given options we have to compare the structures of the given amines as follows:
Structure (A): 2-butanamine

Structure (B): N-ethyl-2-pentanamine

Structure (C): N-methylpiperidine

Structure (D): N,N-dimethylcyclohexylamine

Secondary amines are the amines in which the amino group i.e The nitrogen atom is bonded directly to the two carbon atoms of any hybridization but the carbon atoms must not be carbonyl group carbons.
So, among the given options, structure (A) is eliminated because the nitrogen atom of the amino group is bonded to a single carbon atom only. Structures (C) and (D) are eliminated as well because the nitrogen atom of the amino group in both the compounds are bonded to three carbon atoms i.e., the structure (C) and (D) are the tertiary amines.
In structure B, the nitrogen atom is bonded to two carbon atoms and one hydrogen atom and thus, is a secondary amine which shows positive Liberman-nitroso test. Hence, a positive Libermann-nitroso test will be given by N-ethyl-2-pentanamine.
Therefore, option (B) is the correct answer.
Note: It is important to note that the Libermann-nitroso test is also employed for the test of phenol as on reaction, it forms a deep green or blue colour solution which converts to red on dilution with water.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




