
Which of the following compounds is aspirin?
(A) Methyl salicylate
(B) Acetyl salicylic acid
(C) Phenyl salicylate
(D) Salicylic acid
Answer
233.1k+ views
Hint: (1) Aspirin is an analgesic which means that it can reduce pain without causing any kind of mental confusion or disorder of the nervous system. In other words, it is a neurologically active drug.
(2) The IUPAC name of aspirin is 2-acetoxybenzoic acid.
Complete step by step answer: According to the IUPAC nomenclature of aromatic compounds, the parent hydrocarbon is benzene and when it is monosubstituted by the carboxyl functional group \[\left( {{\text{COOH}}} \right)\] , it is named as benzoic acid. Also, for disubstituted aromatic compounds, the principal group on the ring is given the number 1.
Since the IUPAC name of aspirin is 2-acetoxybenzoic acid, let us first see its structure.
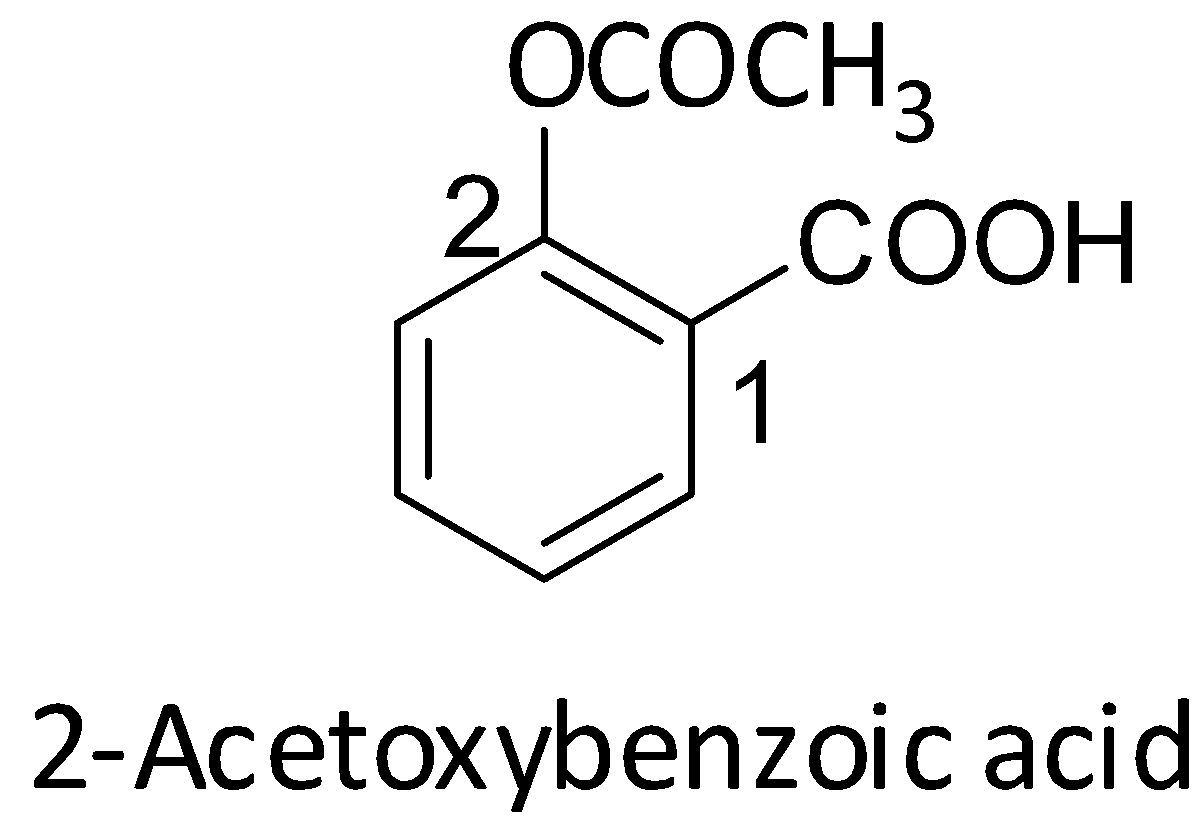
From the structure of aspirin, we can see that it is an acetyl derivative of salicylic acid. It is formed when salicylic acid (2-hydroxybenzoic acid) is treated with acetic anhydride. During this reaction, the hydroxyl group of the salicylic acid$\left( {{\text{OH}}} \right)$ is converted into an ester group $\left( {{\text{OCOC}}{{\text{H}}_{\text{3}}}} \right)$. Acetic acid is a byproduct of the reaction. The formation of aspirin is shown below.

Thus, aspirin is also called acetyl salicylic acid.
So, option B is correct.
Since methyl salicylate is the methyl ester of salicylic acid, its structure will be:
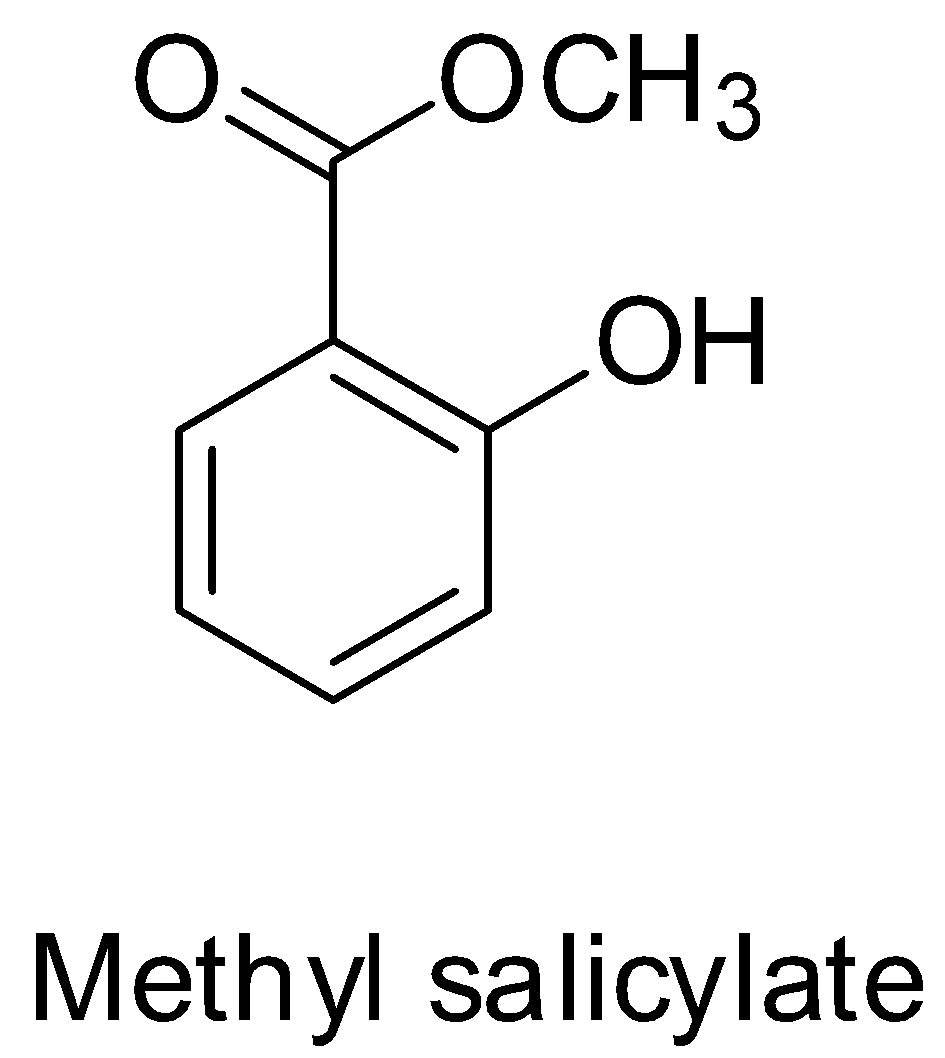
Hence, it does not represent the structure of 2-acetoxybenzoic acid or aspirin. Thus, option A is not correct.
Since phenyl salicylate is the phenyl ester of salicylic acid, its structure will be:
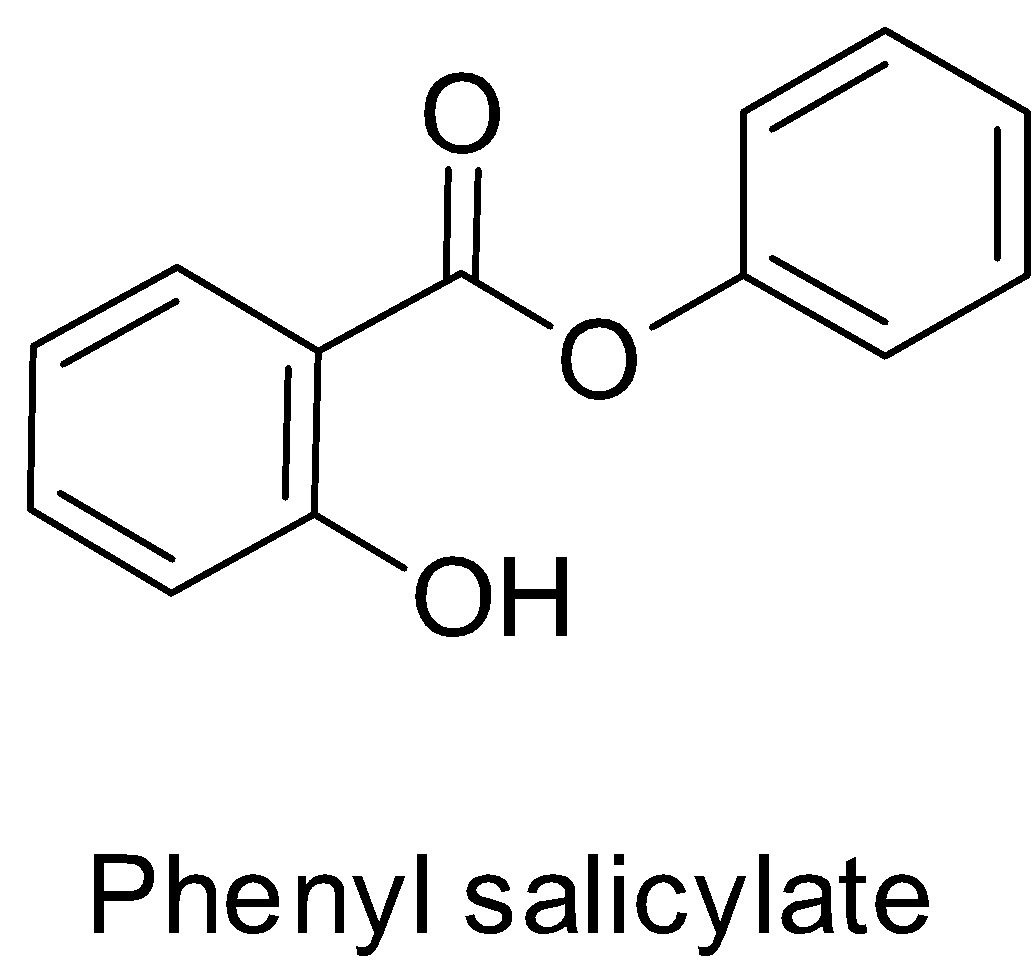
Hence, it does not represent the structure of aspirin or 2-acetoxybenzoic acid. Thus, option C is also not correct.
Lastly, salicylic acid is 2-hydroxybenzoic acid which is used for the synthesis of aspirin. So, it cannot be aspirin itself. Its structure is shown below.
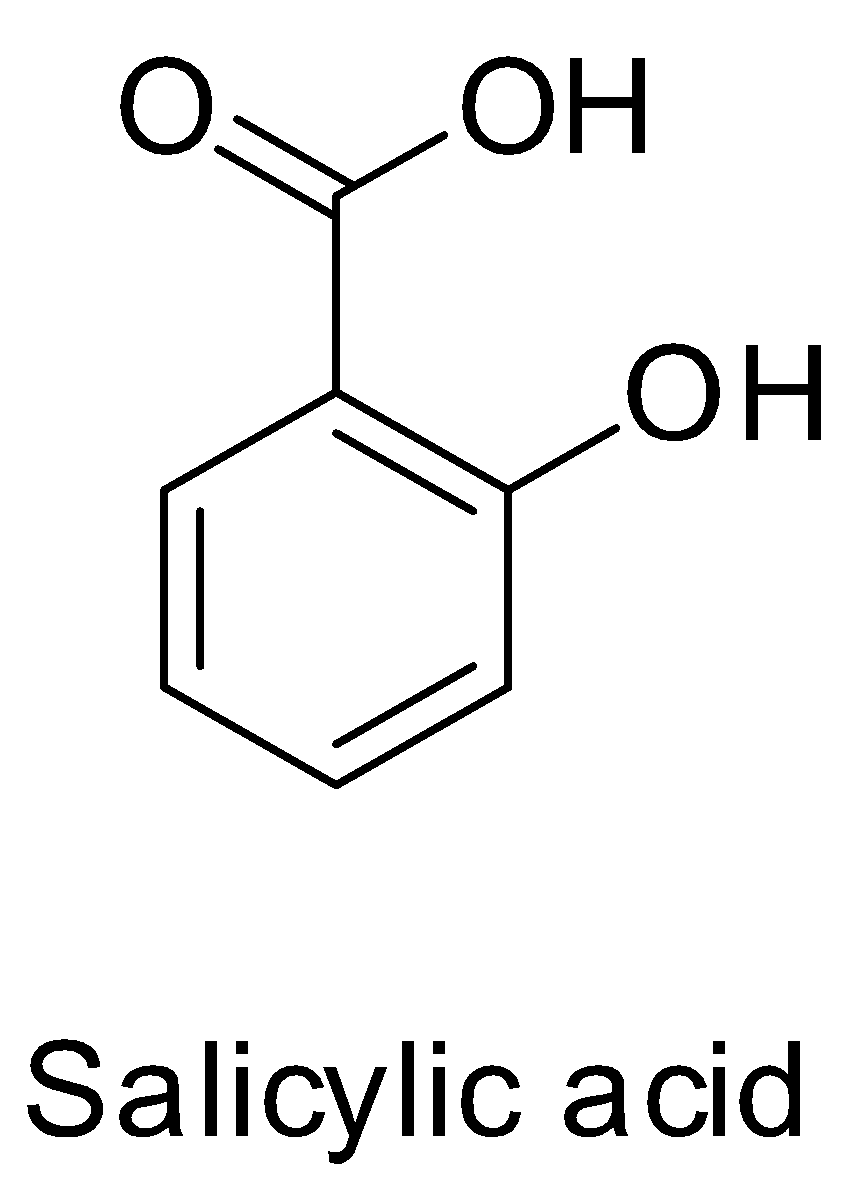
Hence, option D is also not correct.
Note: Aspirin is known to inhibit the synthesis of prostaglandins which causes pain in the tissues. It is also widely used in preventing heart attacks because of its anti-blood clotting action. However, it has some drawbacks too. It is considered to be toxic to liver and sometimes also leads to bleeding from the stomach. Because of these drawbacks, some other analgesics like ibuprofen, naproxen etc. are used as alternatives to aspirin.
(2) The IUPAC name of aspirin is 2-acetoxybenzoic acid.
Complete step by step answer: According to the IUPAC nomenclature of aromatic compounds, the parent hydrocarbon is benzene and when it is monosubstituted by the carboxyl functional group \[\left( {{\text{COOH}}} \right)\] , it is named as benzoic acid. Also, for disubstituted aromatic compounds, the principal group on the ring is given the number 1.

Since the IUPAC name of aspirin is 2-acetoxybenzoic acid, let us first see its structure.

From the structure of aspirin, we can see that it is an acetyl derivative of salicylic acid. It is formed when salicylic acid (2-hydroxybenzoic acid) is treated with acetic anhydride. During this reaction, the hydroxyl group of the salicylic acid$\left( {{\text{OH}}} \right)$ is converted into an ester group $\left( {{\text{OCOC}}{{\text{H}}_{\text{3}}}} \right)$. Acetic acid is a byproduct of the reaction. The formation of aspirin is shown below.

Thus, aspirin is also called acetyl salicylic acid.
So, option B is correct.
Since methyl salicylate is the methyl ester of salicylic acid, its structure will be:

Hence, it does not represent the structure of 2-acetoxybenzoic acid or aspirin. Thus, option A is not correct.
Since phenyl salicylate is the phenyl ester of salicylic acid, its structure will be:

Hence, it does not represent the structure of aspirin or 2-acetoxybenzoic acid. Thus, option C is also not correct.
Lastly, salicylic acid is 2-hydroxybenzoic acid which is used for the synthesis of aspirin. So, it cannot be aspirin itself. Its structure is shown below.

Hence, option D is also not correct.
Note: Aspirin is known to inhibit the synthesis of prostaglandins which causes pain in the tissues. It is also widely used in preventing heart attacks because of its anti-blood clotting action. However, it has some drawbacks too. It is considered to be toxic to liver and sometimes also leads to bleeding from the stomach. Because of these drawbacks, some other analgesics like ibuprofen, naproxen etc. are used as alternatives to aspirin.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




