
Which of the following compounds can give a cannizzaro reaction?
A.
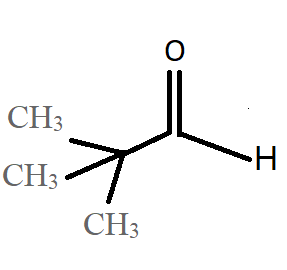
B.
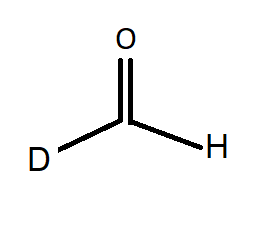
C.
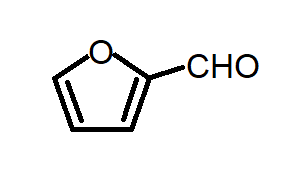
D. All of the above
Answer
233.1k+ views
Hint: Cannizaro reaction is a disproportionation reaction which involves aldehyde with no alpha hydrogen as the reactant. The aldehyde group oxidises and reduces itself to give primary alcohol and a carboxylic acid as major products. Cannizaro reaction takes place in presence of a strong base like $NaOH$followed by \[{H^ + }\]. Cross Cannizaro reaction is not an example of disproportionation but a redox reaction
Complete Step by Step Solution:
Option A: trimethylacetaldehyde or also known as pivalaldehyde has formula \[{C_5}{H_{10}}O\]. Aldehyde group has hydrogen on one side and trimethyl on the other hence, there is no alpha hydrogen. Thus, the product can undergo Cannizaro reaction to form trimethyl acetic acid and 1.1-dimethyl ethanol.
Option B: formaldehyde has chemical formula $HCHO$. Formaldehyde has no alpha hydrogen hence undergoes Cannizaro reaction. If one of the hydrogen is replaced by deuterium then it has no effect on the reaction. Hence the given compound also gives Cannizaro reaction.
Option C: the given compound is known as furfural which is an aldehyde group substituted at the $2$ position of furan. Furfural undergoes Cannizaro reaction and gives furoic acid and furfuryl alcohol as major products.
Option D: thus, all the given compounds give Cannizaro because of absence of any alpha position. So, the correct answer is D.
Note: In the figure below there are two positions from aldehyde group $C = 0$ marked as a and b. The position adjacent to the aldehyde group marked as ‘a’ is known as the alpha position. The next position marked as ‘b’ is known as beta position. Thus, the number of alpha hydrogen in the compound is $5$ and $3$ beta hydrogen.
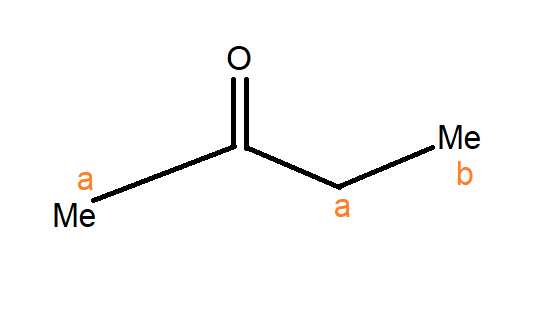
Complete Step by Step Solution:
Option A: trimethylacetaldehyde or also known as pivalaldehyde has formula \[{C_5}{H_{10}}O\]. Aldehyde group has hydrogen on one side and trimethyl on the other hence, there is no alpha hydrogen. Thus, the product can undergo Cannizaro reaction to form trimethyl acetic acid and 1.1-dimethyl ethanol.
Option B: formaldehyde has chemical formula $HCHO$. Formaldehyde has no alpha hydrogen hence undergoes Cannizaro reaction. If one of the hydrogen is replaced by deuterium then it has no effect on the reaction. Hence the given compound also gives Cannizaro reaction.
Option C: the given compound is known as furfural which is an aldehyde group substituted at the $2$ position of furan. Furfural undergoes Cannizaro reaction and gives furoic acid and furfuryl alcohol as major products.
Option D: thus, all the given compounds give Cannizaro because of absence of any alpha position. So, the correct answer is D.
Note: In the figure below there are two positions from aldehyde group $C = 0$ marked as a and b. The position adjacent to the aldehyde group marked as ‘a’ is known as the alpha position. The next position marked as ‘b’ is known as beta position. Thus, the number of alpha hydrogen in the compound is $5$ and $3$ beta hydrogen.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




