
Which doesn’t follow Markovnikov’s rule
A. 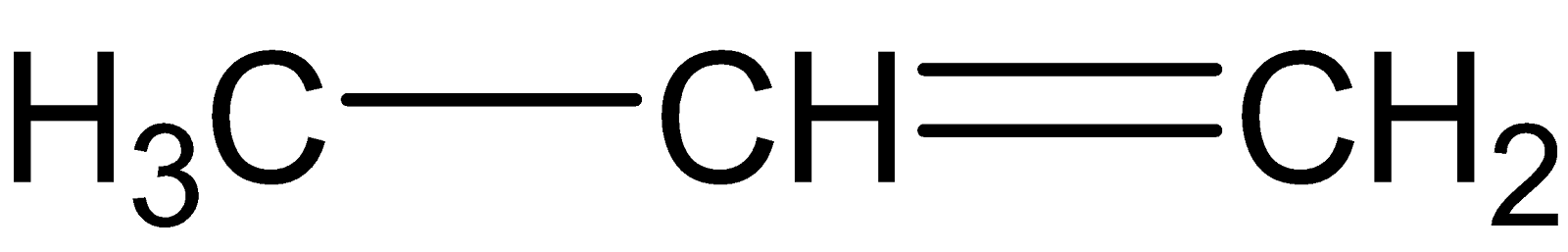
B. 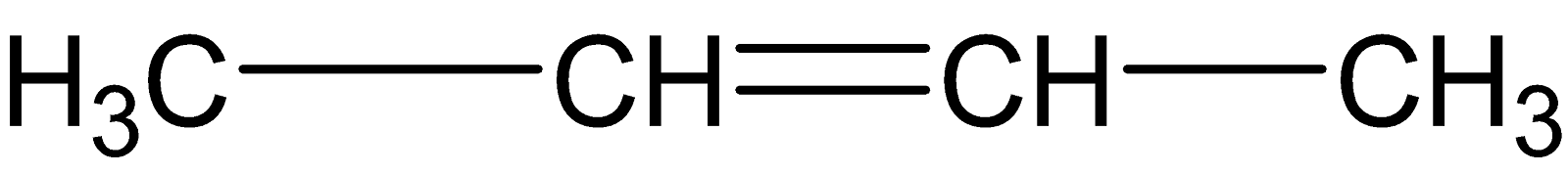
C. 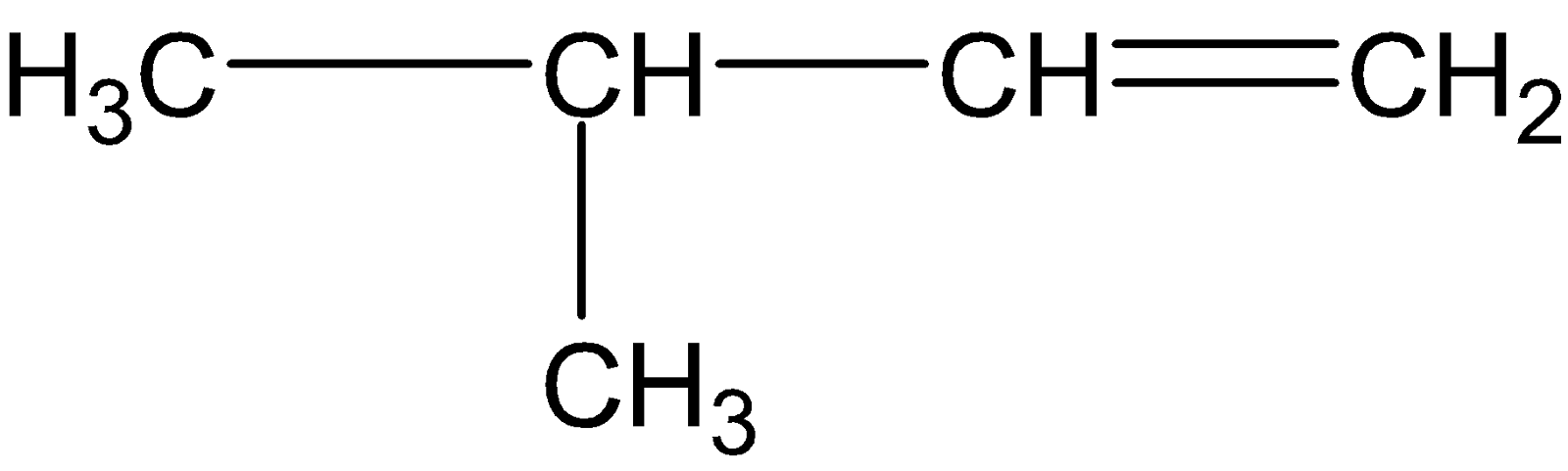
D. 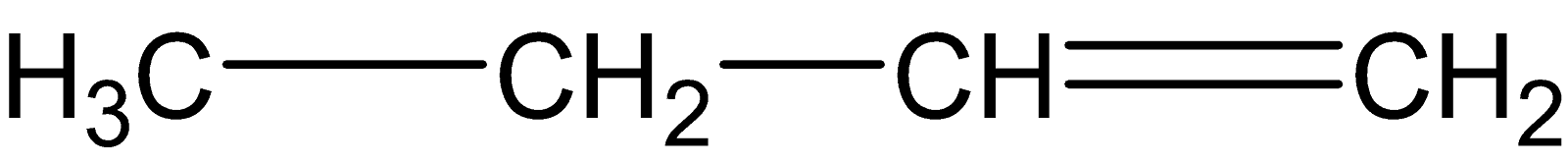
Answer
233.1k+ views
Hint: Markovnikov’s rule is used to determine the regioselectivity of electrophilic addition reactions of unsaturated compounds like alkenes and alkynes. Markovnikov’s rule is only applicable for an asymmetrical unsaturated compound. To approach this question we will have to determine the nature of alkene whether it is symmetric or asymmetric.
Complete Step by Step Answer:
According to Markovnikov’s rule when protic acid reacts with asymmetric alkenes, the positive part of the reagent is added to the carbon atom linked with a higher number of hydrogen atoms while the negative part of the addenda adds to the carbon atom attached with the least number of hydrogen atoms. This electrophilic addition reaction is regioselective in nature.
This rule is only applied to asymmetric alkenes. Now we have to draw the structures to predict their nature.
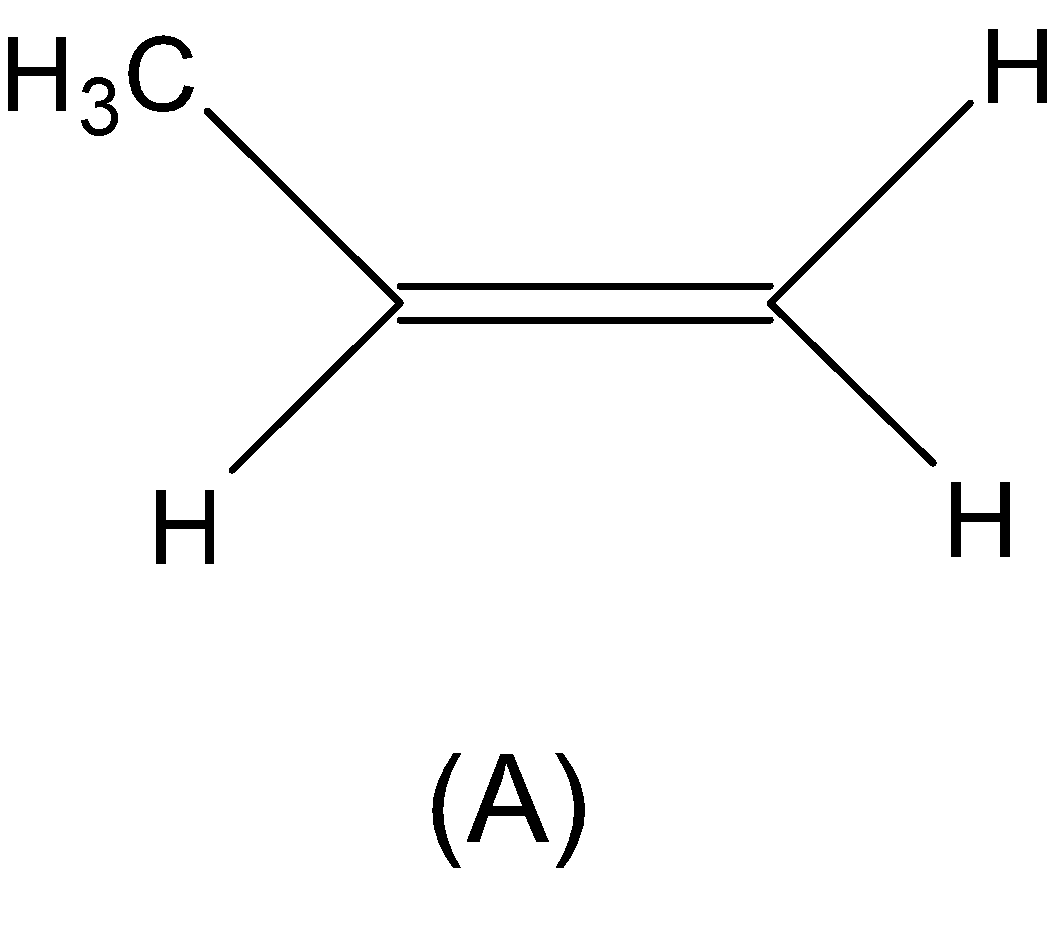
From (A) we have a propene which is symmetric in nature. Hence Markovnikov’s rule is applicable here.
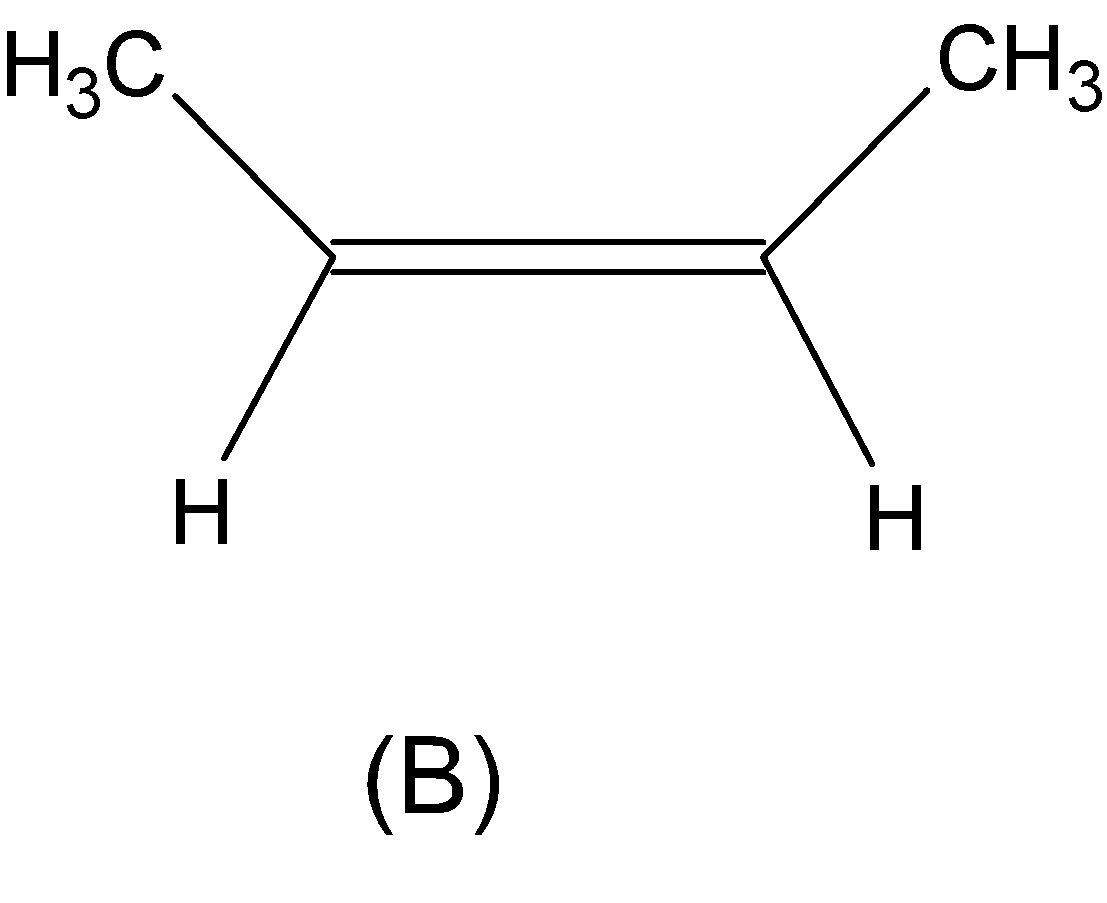
But the alkene which is given in option (B) is not an asymmetric alkene but a symmetric alkene. Thus, Markovnikov’s rule is not applicable here.
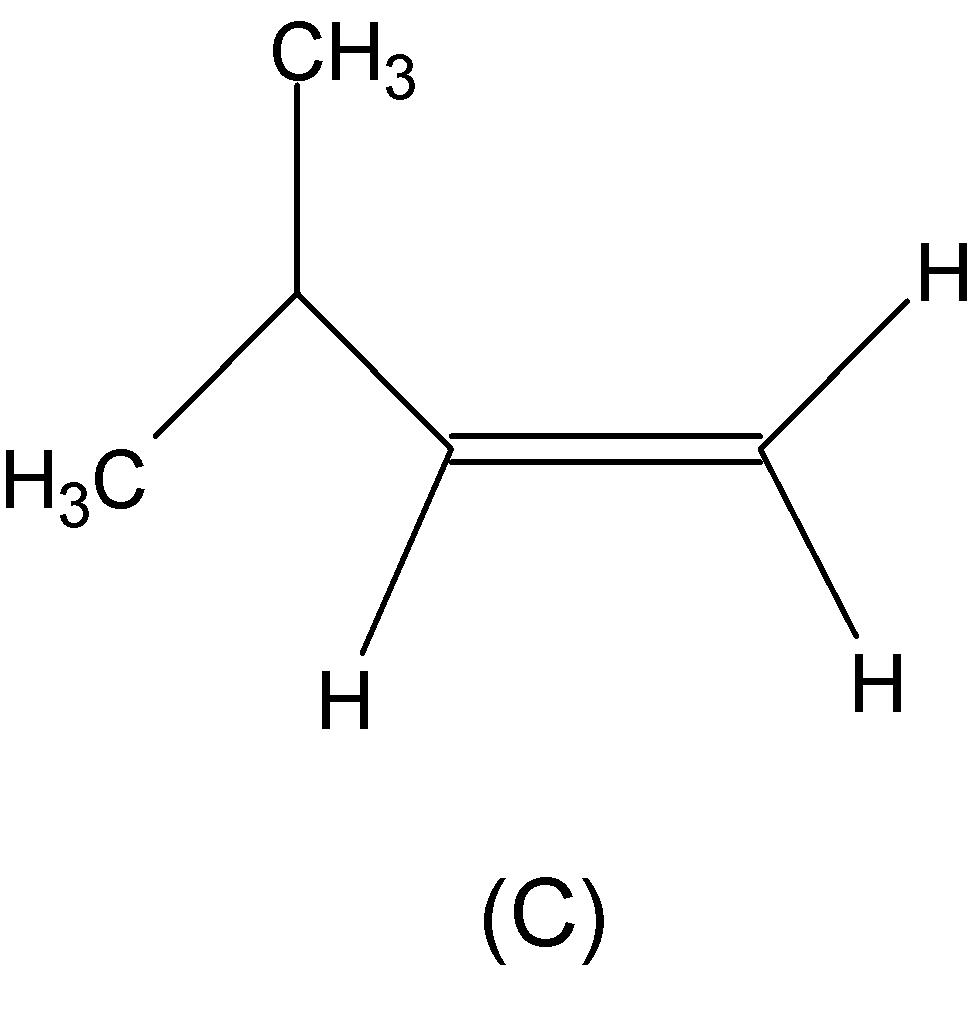
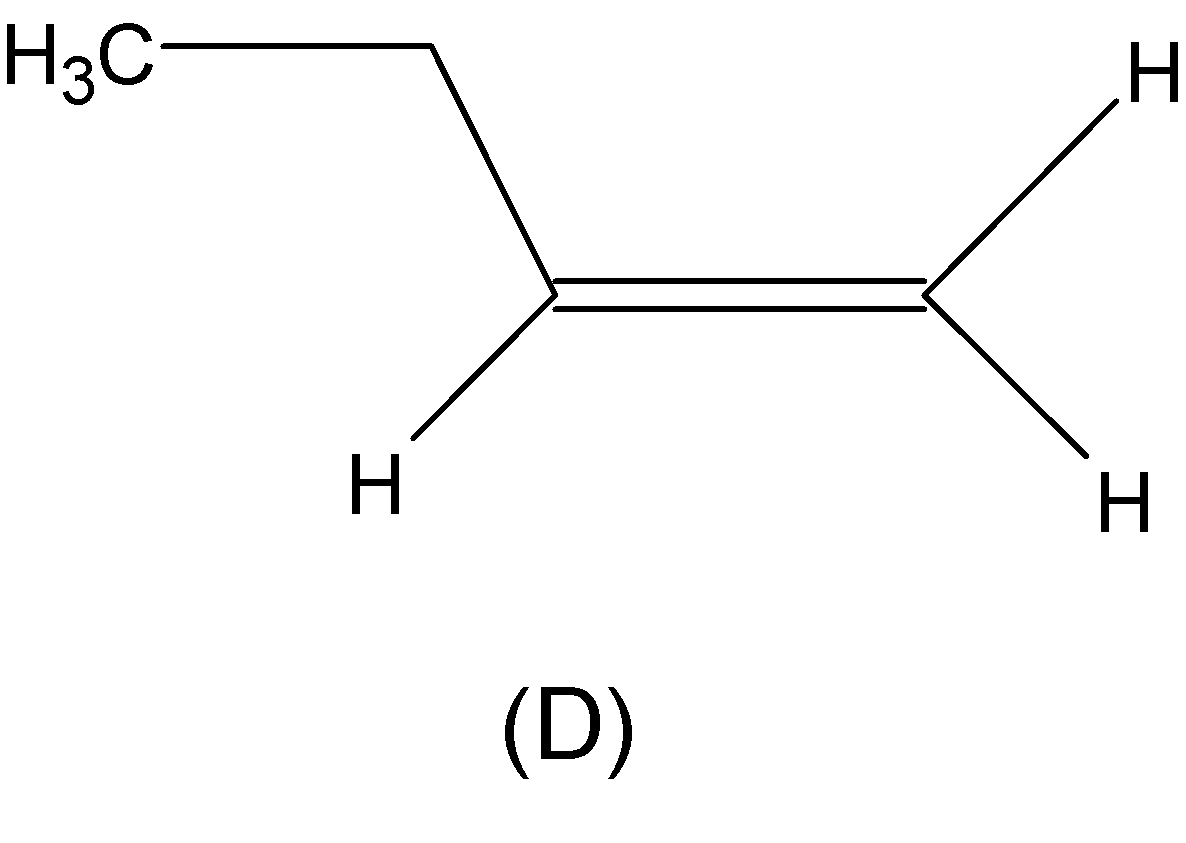
Both the alkenes from (C) and (D) are asymmetric alkenes, thereby undergoing electrophilic addition reaction followed by Markovnikov’s rule.
Thus, option (B) is correct.
Note: The overall regioselectivity will be changed when peroxide is added to the reaction mixtures with protic acid. The major product is formed according to Anti-Markovnikov’s rule. Then this is called the peroxide effect or kharasch effect. The anti-rule is also applicable for asymmetrical alkenes. Among the hydrogen halides hydrogen chloride and hydrogen iodide do not show a peroxide effect.
Complete Step by Step Answer:
According to Markovnikov’s rule when protic acid reacts with asymmetric alkenes, the positive part of the reagent is added to the carbon atom linked with a higher number of hydrogen atoms while the negative part of the addenda adds to the carbon atom attached with the least number of hydrogen atoms. This electrophilic addition reaction is regioselective in nature.
This rule is only applied to asymmetric alkenes. Now we have to draw the structures to predict their nature.
From (A) we have a propene which is symmetric in nature. Hence Markovnikov’s rule is applicable here.

But the alkene which is given in option (B) is not an asymmetric alkene but a symmetric alkene. Thus, Markovnikov’s rule is not applicable here.

Both the alkenes from (C) and (D) are asymmetric alkenes, thereby undergoing electrophilic addition reaction followed by Markovnikov’s rule.


Thus, option (B) is correct.
Note: The overall regioselectivity will be changed when peroxide is added to the reaction mixtures with protic acid. The major product is formed according to Anti-Markovnikov’s rule. Then this is called the peroxide effect or kharasch effect. The anti-rule is also applicable for asymmetrical alkenes. Among the hydrogen halides hydrogen chloride and hydrogen iodide do not show a peroxide effect.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




