
When two soap bubbles of radius ${{r}_{1}}$ and ${{r}_{2}}$ $({{r}_{1}} > {{r}_{2}})$ coalesce, the radius of curvature of common surface is
A . ${{r}_{2}}-{{r}_{1}} \\ $
B . $\dfrac{{{r}_{2}}-{{r}_{1}}}{{{r}_{1}}{{r}_{2}}} \\ $
C . $\dfrac{{{r}_{1}}{{r}_{2}}}{{{r}_{2}}-{{r}_{1}}} \\ $
D . ${{r}_{2}}+{{r}_{1}}$
Answer
233.1k+ views
Hint: Given two soap bubbles with radius ${{r}_{1}}$ and ${{r}_{2}}$ and the two bubbles coalesce means they collide with each other. To solve this question, we use the condition of the excess pressure inside the soap bubble. By taking the two equations with radius ${{r}_{1}}$ and ${{r}_{2}}$ and simplifying it, we get the radius of curvature.
Formula Used:
The excess pressure inside the soap bubble is
$P=\dfrac{4T}{r}$
Where T is the surface tension and R is the radius of the soap bubble.
Complete step by step solution:
Let us consider two soap bubbles of radius ${{r}_{1}}$ and ${{r}_{2}}$ such that $({{r}_{1}}>{{r}_{2}})$
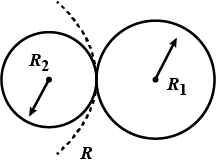
We know the excess pressure inside the soap bubble for radius ${{r}_{1}}$ is
${{P}_{1}}-{{P}_{0}}=\dfrac{4T}{{{r}_{1}}} \\ $
Where T is the surface tension and ${{P}_{1}}$ is the excess pressure inside the first soap bubble and ${{P}_{2}}$ is the excess pressure inside the second soap bubble.
Then ${{P}_{1}}={{P}_{0}}+\dfrac{4T}{{{r}_{1}}} \\ $……………………………………………. (1)
And the excess pressure inside the soap bubble for radius ${{r}_{2}}$is
${{P}_{2}}-{{P}_{0}}=\dfrac{4T}{{{r}_{2}}} \\ $
Then ${{P}_{2}}={{P}_{0}}+\dfrac{4T}{{{r}_{2}}}$…………………………………………. (2)
Let us consider the two bubbles merge
So ${{P}_{1}}-{{P}_{2}}=\dfrac{4T}{r} \\ $
Now we put the value of ${{P}_{1}}$ and ${{P}_{2}}$ in the above equation, we get
${{P}_{0}}+\dfrac{4T}{{{r}_{1}}}-{{P}_{0}}-\dfrac{4T}{{{r}_{2}}}=\dfrac{4T}{r} \\ $
That is $\dfrac{4T}{{{r}_{1}}}-\dfrac{4T}{{{r}_{2}}}=\dfrac{4T}{r} \\ $
Now we take 4T common from LHS, we get
$4T\left( \dfrac{1}{{{r}_{1}}}-\dfrac{1}{{{r}_{2}}} \right)=\dfrac{4T}{r} \\ $
Now we cancel 4T from both sides and get
$\left( \dfrac{1}{{{r}_{1}}}-\dfrac{1}{{{r}_{2}}} \right)=\dfrac{1}{r} \\ $
Then $r=\dfrac{{{r}_{1}}{{r}_{2}}}{{{r}_{2}}-{{r}_{1}}} \\ $
Therefore, the radius of curvature of common surface is $r=\dfrac{{{r}_{1}}{{r}_{2}}}{{{r}_{2}}-{{r}_{1}}}$
Thus, option C is the correct answer.
Note: Surface tension is the film of a liquid which occurs on the surface because of the attraction of the surface particles by the liquid that tries to minimise the surface area of liquid drop. This phenomenon is called surface tension.
Formula Used:
The excess pressure inside the soap bubble is
$P=\dfrac{4T}{r}$
Where T is the surface tension and R is the radius of the soap bubble.
Complete step by step solution:
Let us consider two soap bubbles of radius ${{r}_{1}}$ and ${{r}_{2}}$ such that $({{r}_{1}}>{{r}_{2}})$

We know the excess pressure inside the soap bubble for radius ${{r}_{1}}$ is
${{P}_{1}}-{{P}_{0}}=\dfrac{4T}{{{r}_{1}}} \\ $
Where T is the surface tension and ${{P}_{1}}$ is the excess pressure inside the first soap bubble and ${{P}_{2}}$ is the excess pressure inside the second soap bubble.
Then ${{P}_{1}}={{P}_{0}}+\dfrac{4T}{{{r}_{1}}} \\ $……………………………………………. (1)
And the excess pressure inside the soap bubble for radius ${{r}_{2}}$is
${{P}_{2}}-{{P}_{0}}=\dfrac{4T}{{{r}_{2}}} \\ $
Then ${{P}_{2}}={{P}_{0}}+\dfrac{4T}{{{r}_{2}}}$…………………………………………. (2)
Let us consider the two bubbles merge
So ${{P}_{1}}-{{P}_{2}}=\dfrac{4T}{r} \\ $
Now we put the value of ${{P}_{1}}$ and ${{P}_{2}}$ in the above equation, we get
${{P}_{0}}+\dfrac{4T}{{{r}_{1}}}-{{P}_{0}}-\dfrac{4T}{{{r}_{2}}}=\dfrac{4T}{r} \\ $
That is $\dfrac{4T}{{{r}_{1}}}-\dfrac{4T}{{{r}_{2}}}=\dfrac{4T}{r} \\ $
Now we take 4T common from LHS, we get
$4T\left( \dfrac{1}{{{r}_{1}}}-\dfrac{1}{{{r}_{2}}} \right)=\dfrac{4T}{r} \\ $
Now we cancel 4T from both sides and get
$\left( \dfrac{1}{{{r}_{1}}}-\dfrac{1}{{{r}_{2}}} \right)=\dfrac{1}{r} \\ $
Then $r=\dfrac{{{r}_{1}}{{r}_{2}}}{{{r}_{2}}-{{r}_{1}}} \\ $
Therefore, the radius of curvature of common surface is $r=\dfrac{{{r}_{1}}{{r}_{2}}}{{{r}_{2}}-{{r}_{1}}}$
Thus, option C is the correct answer.
Note: Surface tension is the film of a liquid which occurs on the surface because of the attraction of the surface particles by the liquid that tries to minimise the surface area of liquid drop. This phenomenon is called surface tension.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




