
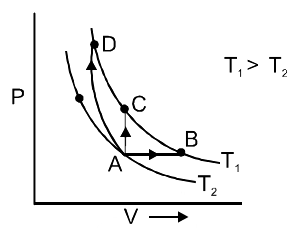
Three different processes that can occur in an ideal monatomic gas are shown in the P vs V diagram. The paths are labelled as A → B, A → C and A → D. The change in internal energies during these processes are taken as ${E_{AB}}$ , ${E_{AC}}$ and ${E_{AD}}$ and the work done as ${W_{AB}}$ , ${W_{AC}}$ and ${W_{AD}}. The correct relation between these parameters are:
1) ${E_{AB}} = {E_{AC}} = {E_{AD}}, {W_{AB}} > 0, {W_{AC}} = 0, {W_{AD}} < 0$
2) ${E_{AB}} > {E_{AC}} > {E_{AD}} {W_{AB}} < {W_{AC}} < {W_{AD}} $
3) ${E_{AB}} < {E_{AC}} < {E_{AD}} {W_{AB}} > 0, {W_{AC}} > {W_{AD}}$
4)$ {E_{AB}} = {E_{AC}} = {E_{AD}} {W_{AB}} > 0, {W_{AC}} = 0, {W_{AD}} > 0$
Answer
232.8k+ views
Hint: When a monatomic gas behaves optimally, that is, when the gas perfectly obeys the ideal gas equation PV=nRT, it is referred to as an ideal monatomic gas. In thermodynamics, a monatomic gas is one in which the atoms of the gas are not bound to each other such that gas consists of multiple single atoms of particular type. Here, we'll look at the graphed relationship between P and V to get the molar heat capacity.
Complete answer:
The sum of all the energy attributed to the motion of the atoms or molecules within the system is known as internal energy. The rotation, vibration, translation, and interactions of a substance's molecules are examples of microscopic kinds of energy.
Monatomic Gas – Internal energy:
The only source of energy in a monatomic ideal gas (such as helium, neon, or argon) is translational kinetic energy. The equation states that the average translational kinetic energy of a single atom depends only on the temperature of the gas:
${{K}_{avg}}=\dfrac {3}{2} kT$
The average kinetic energy per molecule multiplied by the overall number of molecules, N, gives the internal energy of n moles of an ideal monatomic (one atom per molecule) gas.
\[{{E}_{int}}\text{ }=\text{ }3/2\text{ }NkT\text{ }=\text{ }3/2\text{ }nRT\]
The internal energy is contributed by the three directions (x, y, and z), where n is the number of moles. This is where the concept of energy partition enters; any additional energy supply must also provide (1/2)nRT. As shown, the internal energy of an ideal gas only varies with temperature and the quantity of gas molecules.
We know that the initial and final temperatures for each of the processes are the same.
Therefore, change in internal energy for each process will be equal, i.e.,
$\Delta U=n{{C}_{v}}\Delta T=same$
Thus, ${{E}_{AB}}={{E}_{AC}}={{E}_{AD}}$
Now, work done is given by,
$W=pdV$
For process AB. Volume is increasing
$\Rightarrow {{W}_{AB}}>0$
For process AD, volume is decreasing
$\Rightarrow {{W}_{AD}}<0$
For process AC, volume is constant
$\Rightarrow {{W}_{AC}}=0$
Hence, the option (1) is correct.
Note:These atoms are stable. They rarely form compounds with other atoms because they have fuller outer energy levels. Because it is simple to separate them by overcoming the weak forces of attraction between the atoms, they have low melting and boiling temperatures.
Complete answer:
The sum of all the energy attributed to the motion of the atoms or molecules within the system is known as internal energy. The rotation, vibration, translation, and interactions of a substance's molecules are examples of microscopic kinds of energy.
Monatomic Gas – Internal energy:
The only source of energy in a monatomic ideal gas (such as helium, neon, or argon) is translational kinetic energy. The equation states that the average translational kinetic energy of a single atom depends only on the temperature of the gas:
${{K}_{avg}}=\dfrac {3}{2} kT$
The average kinetic energy per molecule multiplied by the overall number of molecules, N, gives the internal energy of n moles of an ideal monatomic (one atom per molecule) gas.
\[{{E}_{int}}\text{ }=\text{ }3/2\text{ }NkT\text{ }=\text{ }3/2\text{ }nRT\]
The internal energy is contributed by the three directions (x, y, and z), where n is the number of moles. This is where the concept of energy partition enters; any additional energy supply must also provide (1/2)nRT. As shown, the internal energy of an ideal gas only varies with temperature and the quantity of gas molecules.
We know that the initial and final temperatures for each of the processes are the same.
Therefore, change in internal energy for each process will be equal, i.e.,
$\Delta U=n{{C}_{v}}\Delta T=same$
Thus, ${{E}_{AB}}={{E}_{AC}}={{E}_{AD}}$
Now, work done is given by,
$W=pdV$
For process AB. Volume is increasing
$\Rightarrow {{W}_{AB}}>0$
For process AD, volume is decreasing
$\Rightarrow {{W}_{AD}}<0$
For process AC, volume is constant
$\Rightarrow {{W}_{AC}}=0$
Hence, the option (1) is correct.
Note:These atoms are stable. They rarely form compounds with other atoms because they have fuller outer energy levels. Because it is simple to separate them by overcoming the weak forces of attraction between the atoms, they have low melting and boiling temperatures.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




