
The structure of cis-bis(propenyl) ethene is:
(a) 
(b) 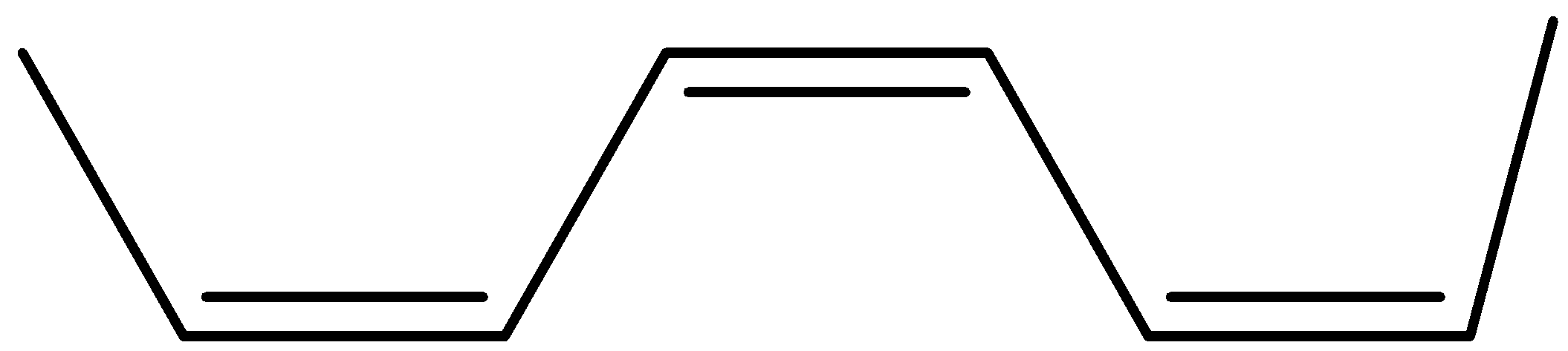
(c) 
(d)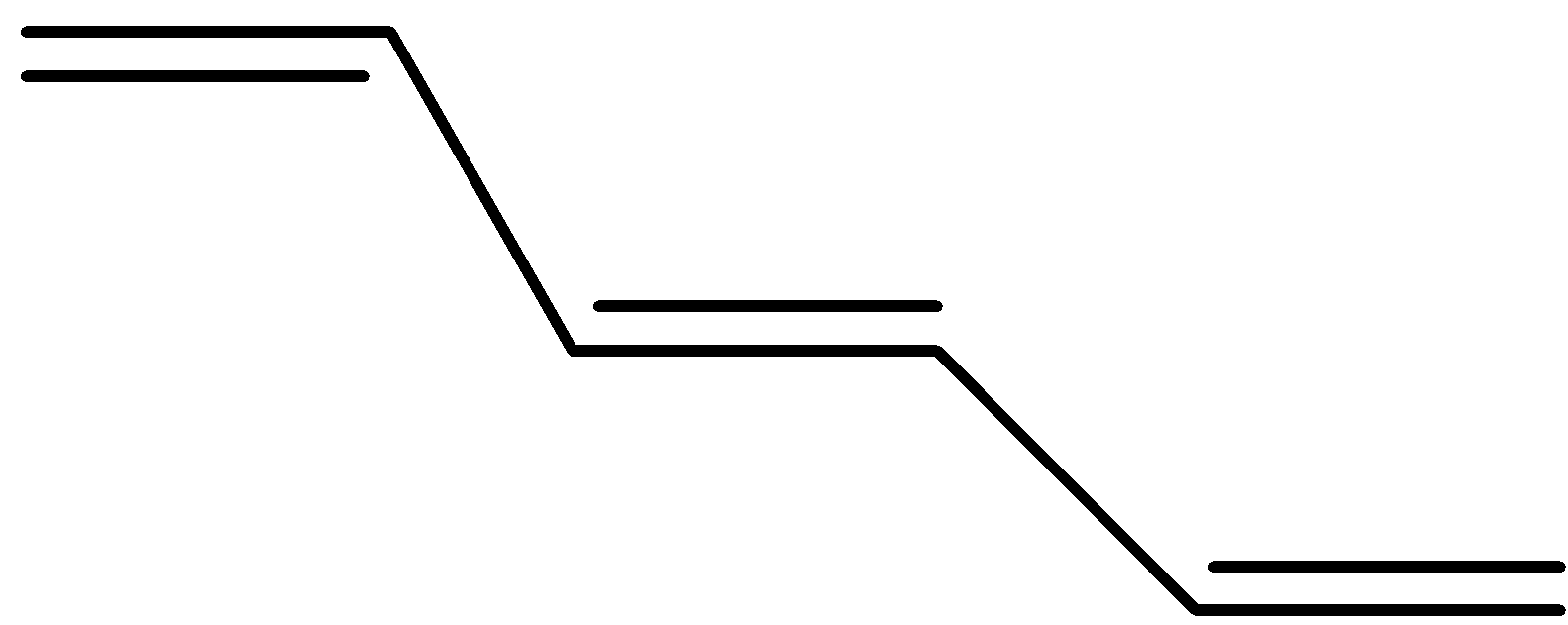
Answer
232.8k+ views
Hint:Cis isomer is where the same groups are on same side of an unsaturated bond. Trans isomer is when the same groups are on opposite sides of an unsaturated bond. This is only in the case of organic compounds.
Complete step by step answer:
The structure of bis(propenyl) ethene is
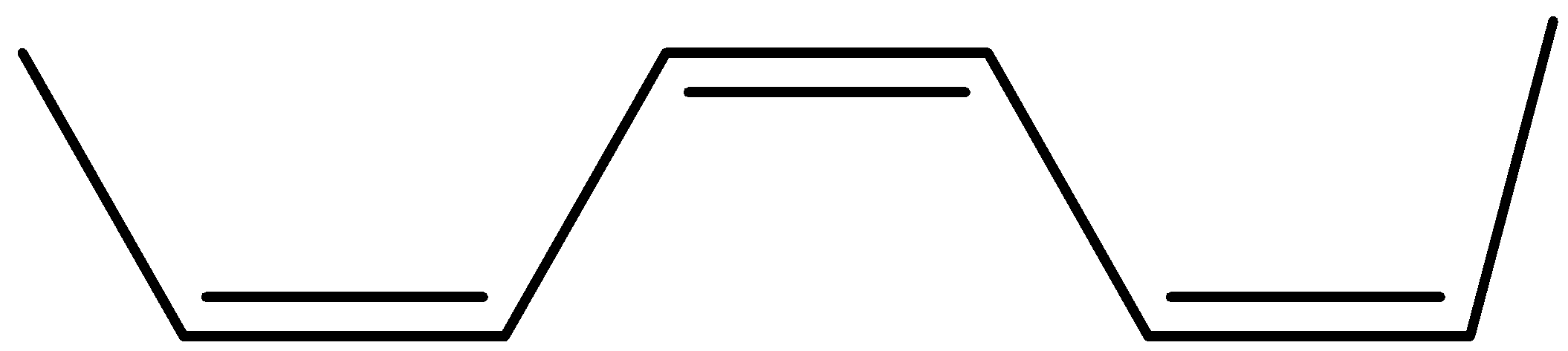
Now its cis isomer is where the 2 hydrogen will be on the same side and the alkyl groups on the other side.
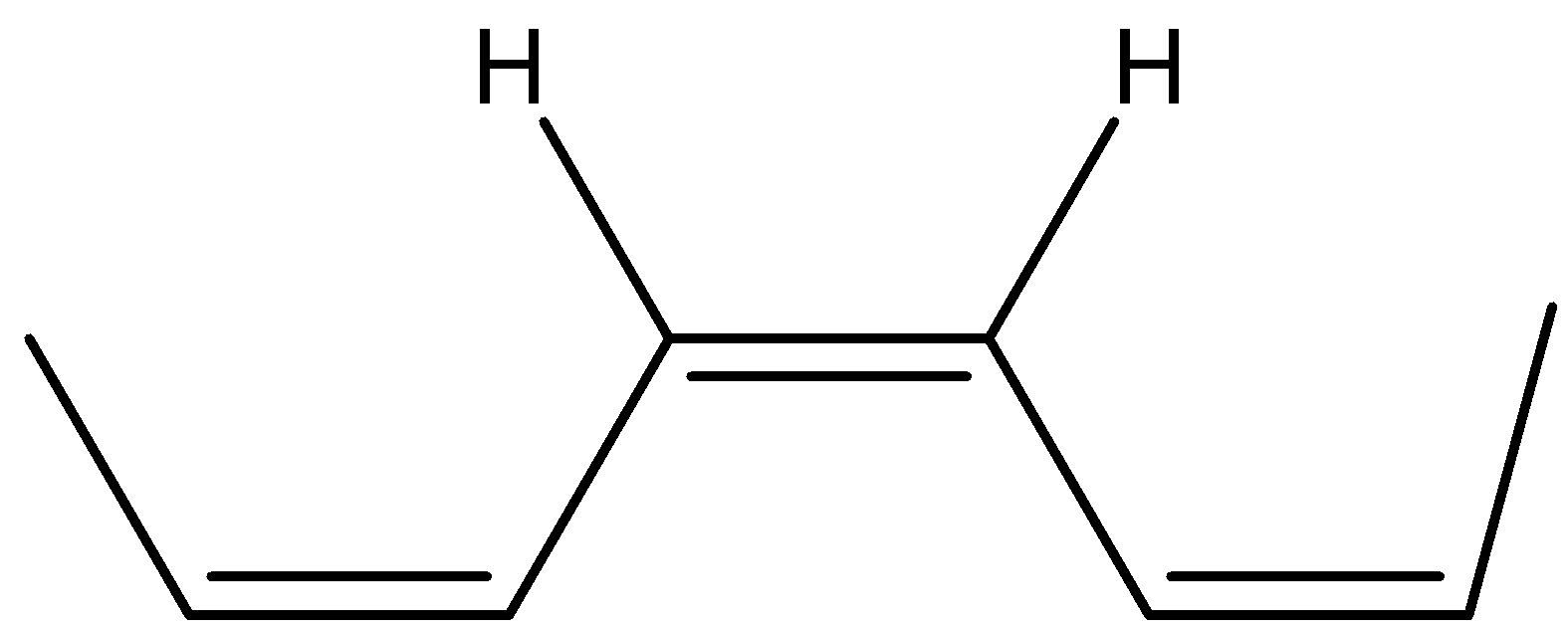
The trans form the bis(propenyl) ethene will be where the hydrogen are in opposite sides or the alkyl groups are on opposite sides and its structure will be as shown below
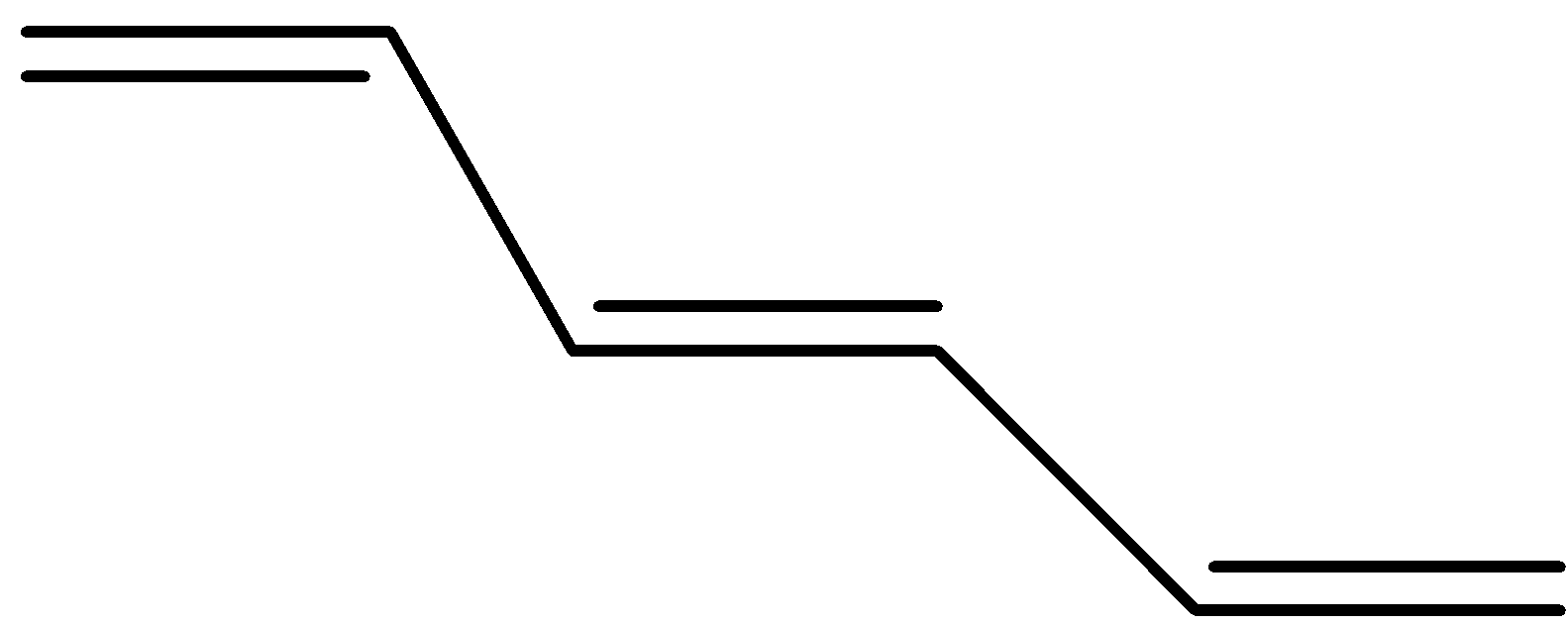
Hence, the correct answer to the question is option (b).
Additional Information:
Cis-Trans isomerism or the geometrical isomerism is also known as configurationally isomerism. Cis indicated that the functional groups are on the same side of the carbon chain while trans means that they are on opposing sides of the carbon chains. Cis and trans isomers are stereoisomer. These are pairs of molecules having the same formula but the functional groups are rotated into different orientations in three-dimensional space. This type of isomerism occurs in both organic and inorganic molecules. In inorganic molecules, it is seen in complexes.
Note:
Cis and trans isomers can only be formed if there is an unsaturated bond, that is, a double bond or a triple bond. It is an arrangement of the atoms around the double or a triple bond. Only in the case of organic compounds.
Complete step by step answer:
The structure of bis(propenyl) ethene is

Now its cis isomer is where the 2 hydrogen will be on the same side and the alkyl groups on the other side.

The trans form the bis(propenyl) ethene will be where the hydrogen are in opposite sides or the alkyl groups are on opposite sides and its structure will be as shown below

Hence, the correct answer to the question is option (b).
Additional Information:
Cis-Trans isomerism or the geometrical isomerism is also known as configurationally isomerism. Cis indicated that the functional groups are on the same side of the carbon chain while trans means that they are on opposing sides of the carbon chains. Cis and trans isomers are stereoisomer. These are pairs of molecules having the same formula but the functional groups are rotated into different orientations in three-dimensional space. This type of isomerism occurs in both organic and inorganic molecules. In inorganic molecules, it is seen in complexes.
Note:
Cis and trans isomers can only be formed if there is an unsaturated bond, that is, a double bond or a triple bond. It is an arrangement of the atoms around the double or a triple bond. Only in the case of organic compounds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




