
The reduction of which of the following compounds would yield secondary amine?
A. Alkyl nitrile
B. Carbylamine
C. Primary amine
D. Secondary nitro compounds
Answer
233.1k+ views
Hint: Reduction is the addition of hydrogen or removal of oxygen from the compounds. In secondary amine, two alkyl groups of any hybridization are attached to the nitrogen of the amine group.
Complete Step by Step Solution:
In the first option, alkyl nitrile gives primary amines on reduction. In option C, primary amine will not reduce at all as it is a reduced product of amide. In option D, secondary nitro compounds reduce to give primary amine. Carbylamine, also called isocyanide, gives secondary amine on reduction. Carbylamine is a primary amine. It has an isocyanate functional group in which one pie bond breaks and hydrogen gets attached to the nitrogen and carbon atom of the isocyanide group to give an amine functional group where nitrogen is attached to two carbons. Reduction of carbylamine can be done in presence of hydrogen gas and any metal like nickel or platinum and this takes place as follows:
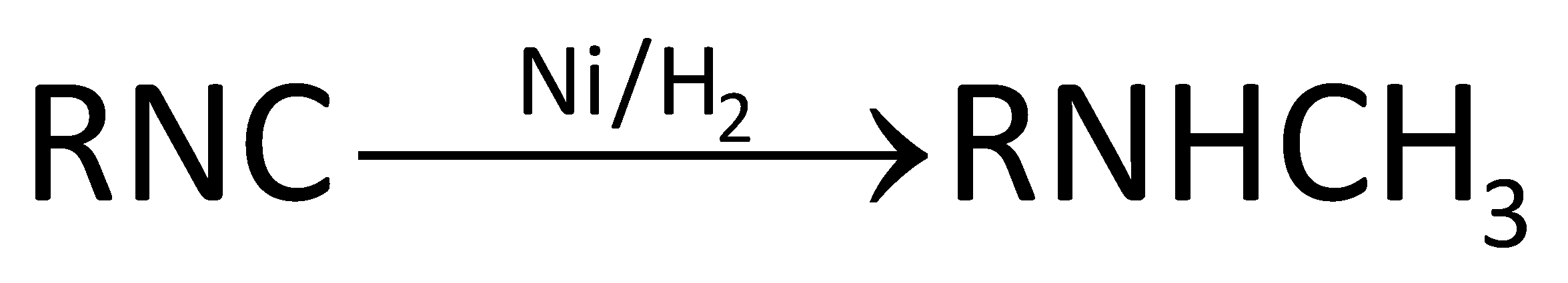
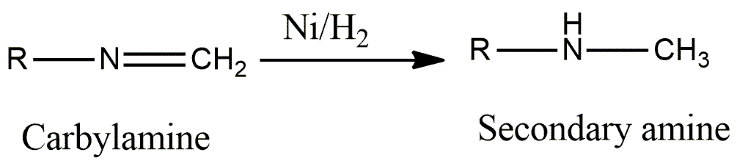
Image:Reduction of carbylamine
So, option B is correct.
Note: Secondary nitro compounds are those which have two carbons attached to the carbon which is adjacent to the nitrogen of the nitro functional group. On reduction, the oxygen of nitro groups gets removed and two hydrogens get attached to the Nitrogen of the nitro functional group. So, the product will have only one carbon attached to the nitrogen of the amine group which is a primary amine.
Complete Step by Step Solution:
In the first option, alkyl nitrile gives primary amines on reduction. In option C, primary amine will not reduce at all as it is a reduced product of amide. In option D, secondary nitro compounds reduce to give primary amine. Carbylamine, also called isocyanide, gives secondary amine on reduction. Carbylamine is a primary amine. It has an isocyanate functional group in which one pie bond breaks and hydrogen gets attached to the nitrogen and carbon atom of the isocyanide group to give an amine functional group where nitrogen is attached to two carbons. Reduction of carbylamine can be done in presence of hydrogen gas and any metal like nickel or platinum and this takes place as follows:
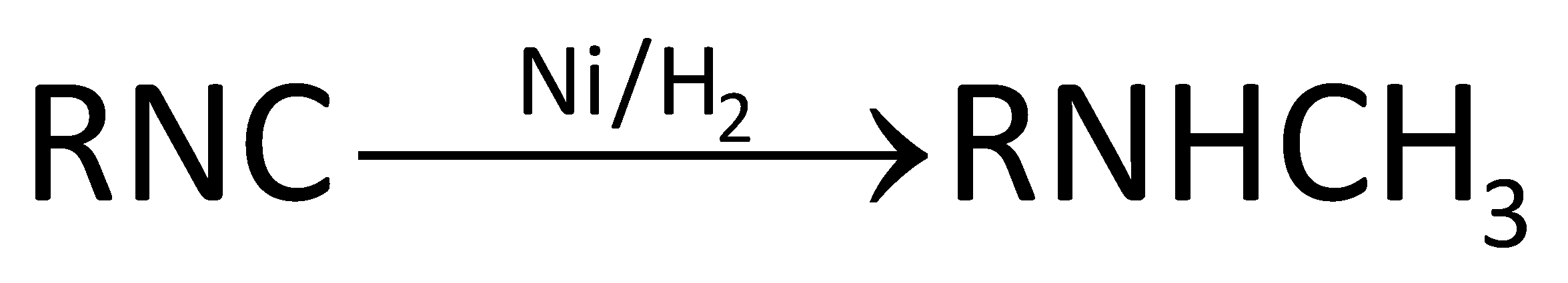
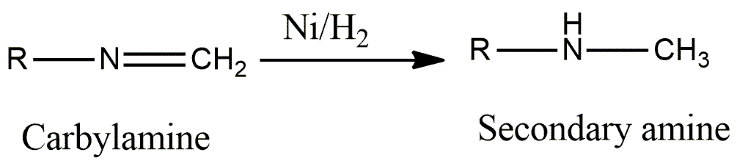
Image:Reduction of carbylamine
So, option B is correct.
Note: Secondary nitro compounds are those which have two carbons attached to the carbon which is adjacent to the nitrogen of the nitro functional group. On reduction, the oxygen of nitro groups gets removed and two hydrogens get attached to the Nitrogen of the nitro functional group. So, the product will have only one carbon attached to the nitrogen of the amine group which is a primary amine.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




