
The reactivity of halogen atom is minimum in
(a) Propyl chloride
(b) Propyl iodide
(c) Isopropyl chloride
(d) Isopropyl bromide
Answer
232.8k+ views
Hint: We know that halogens belong to the 17th group of the periodic table. The halogens are chlorine, iodine, bromine, fluorine, and iodine. The reactivity of halogens is very high and fluorine has the highest electronegativity.
Complete Step by Step Solution:
Let's discuss the reactivity of the halogens. The leaving ability of the halogens decides the reaction rate. And the order of leaving halogens is: Iodine>Bromine>Chlorine>Fluorine. Also, the leaving ability is dependent on the degree of the halogens. The leaving ability order is \[1^\circ > 2^\circ > 3^\circ \].
Here, four alkyl halides are given whose names are propyl iodide, propyl chloride, isopropyl bromide and isopropyl bromide. Let's draw the structures of these alkyl halides.
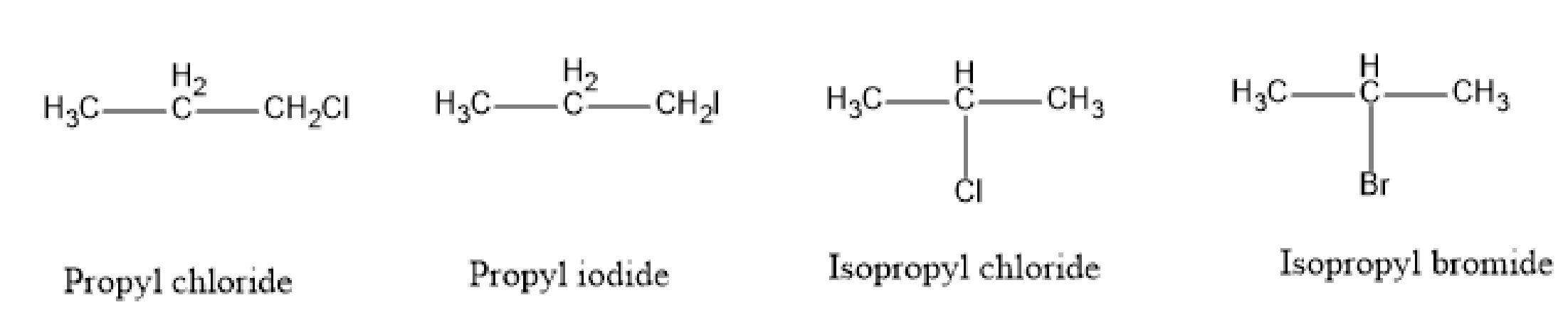
Fig:Alkyl halides
We know that iodine is the best leaving group. Therefore, propyl iodide is the most reactive among the given alkyl halides.
After iodine, bromine is the good leaving group. Therefore, isopropyl bromide is most reactive from the remaining three alkyl halides.
Now, we have to compare the reactivity of propyl chloride and isopropyl chloride. From the structure, we know that propyl chloride is \[1^\circ \] and isopropyl chloride is \[2^\circ \] carbocation. And we know, reactivity order, that is,\[1^\circ > 2^\circ > 3^\circ \] .
Therefore, isopropyl chloride is the least reactive. Hence, option c is right.
Note: Halogens are the most electronegative elements because they contain seven electrons in the valence shell and thus require only one electron to attain stability. The order of halogens in the electronegativity trend is F > Cl > Br > I.
Complete Step by Step Solution:
Let's discuss the reactivity of the halogens. The leaving ability of the halogens decides the reaction rate. And the order of leaving halogens is: Iodine>Bromine>Chlorine>Fluorine. Also, the leaving ability is dependent on the degree of the halogens. The leaving ability order is \[1^\circ > 2^\circ > 3^\circ \].
Here, four alkyl halides are given whose names are propyl iodide, propyl chloride, isopropyl bromide and isopropyl bromide. Let's draw the structures of these alkyl halides.

Fig:Alkyl halides
We know that iodine is the best leaving group. Therefore, propyl iodide is the most reactive among the given alkyl halides.
After iodine, bromine is the good leaving group. Therefore, isopropyl bromide is most reactive from the remaining three alkyl halides.
Now, we have to compare the reactivity of propyl chloride and isopropyl chloride. From the structure, we know that propyl chloride is \[1^\circ \] and isopropyl chloride is \[2^\circ \] carbocation. And we know, reactivity order, that is,\[1^\circ > 2^\circ > 3^\circ \] .
Therefore, isopropyl chloride is the least reactive. Hence, option c is right.
Note: Halogens are the most electronegative elements because they contain seven electrons in the valence shell and thus require only one electron to attain stability. The order of halogens in the electronegativity trend is F > Cl > Br > I.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




