
What would be the product formed when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether?
A . 1-Bromocyclobutane
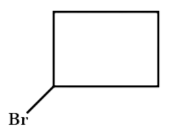
B . 1 - Chlorocyclobutane
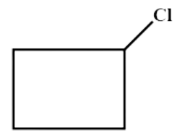
C . Cyclobutane

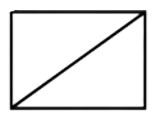
D . Bicyclo ( 1 , 1 , 0 ) ) butane
Answer
241.2k+ views
Hint: In this question, intramolecular Wurtz reaction is taking place. With the help of radical species R, the Wurtz reaction entails the transfer of halogen and metal in order to create a carbon-carbon bond that results from a nucleophilic substitution process. The process involves reacting alkyl halides with metallic sodium in the presence of dry ether to produce higher alkanes.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
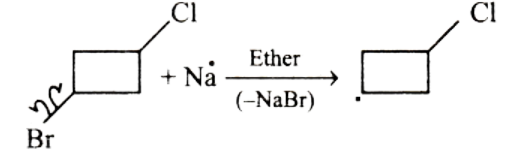
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.
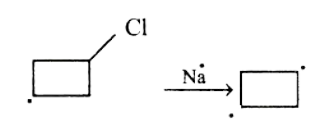
The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:
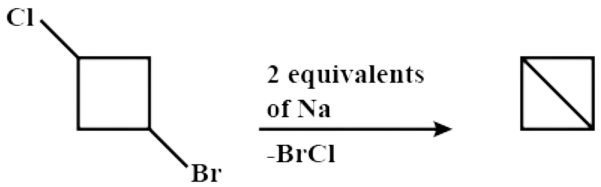
Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
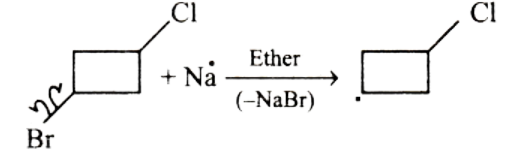
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.

The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:

Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




