
The number of stereoisomers possible for \[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\] is ____________. [ox = oxalate]
Answer
233.1k+ views
Hint: There are two types of isomers- structural and stereoisomer. Stereoisomers are formed due to change in spatial arrangement of atoms. This spatial arrangement may lead to formation of optically active compounds or optically inactive compounds. The compounds which have non-superimposable mirror images are termed as optically active or chiral compounds. Whereas those which do not form a non-superimposable mirror image are termed as optically inactive or achiral compounds.
Complete step-by-step answer:In the given compound\[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\].
Co is the central metal atom whereas \[ox,{\text{ }}Br,{\text{ }}N{H_3}\] are the ligands. The compound is of the form \[\left[ {A{B_2}CD} \right]\] with total no. of ligands=\[4\].
Oxalate or \[ox\] is a bidentate ligand. Hence it coordinates to the central metal atom using two sites. ‘Bi’ means two and ‘dentate’ refers to the denticity. \[Br\] and \[N{H_3}\]are mondentate ligands. Hence total number of coordination sites\[ = 2\left( 2 \right){\text{ }} + 1 + 1 = 6\].
Hence coordination number of compound is \[6\]. Thus, the geometry and shape of the complex is octahedral.
Drawing different structures of given compound by changing the position of ligands result in two structures as shown below.
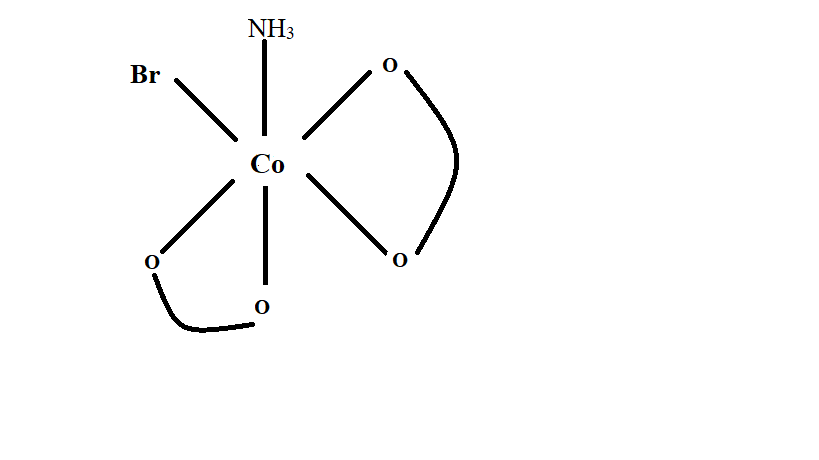
Image: Isomers-1 of \[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\]
In the above image, the compound has a non-superimposable mirror image hence the mirror image is considered as a different stereoisomer of the compound.
Thus number of stereoisomer from isomers (1) is \[2\].
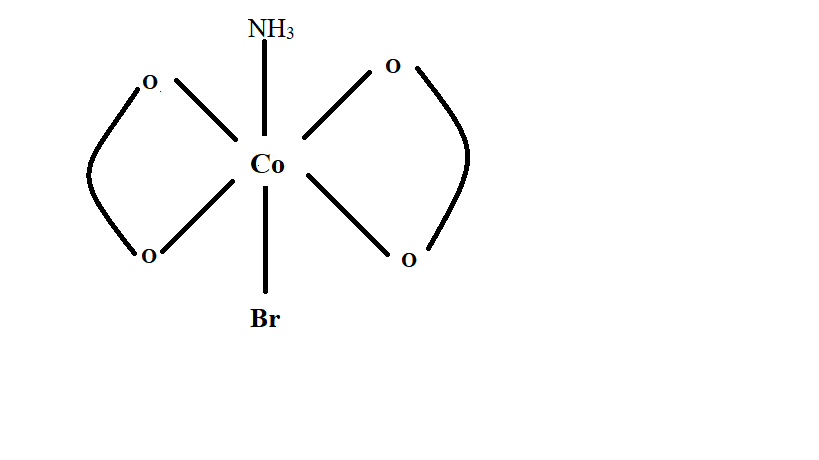
Image: Isomers-2 of \[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\]
In the above image, the compound has a plane of symmetry passing through the center of the compound with one oxalate ligand reflecting other and \[Br\] and \[N{H_3}\] bisecting. Hence the mirror image is same as that of the original structure and thus, not considered a different compound.
Thus, number of stereoisomer from isomer (2) is 1
Total number of stereoisomer of \[\mathbf{{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;}\]\[\mathbf{ = 2 + 1 = 3}\]
Note:Denticity means the number of donor sites available to directly attach with central metal atom.
There are various symmetry elements like plane of symmetry, center of symmetry, etc. used to describe the chirality or optical activity of the compound. If a compound has either plane of symmetry or center of symmetry then it is said to be optically inactive or achiral and no non superimposable mirror image of the compound exists.
Complete step-by-step answer:In the given compound\[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\].
Co is the central metal atom whereas \[ox,{\text{ }}Br,{\text{ }}N{H_3}\] are the ligands. The compound is of the form \[\left[ {A{B_2}CD} \right]\] with total no. of ligands=\[4\].
Oxalate or \[ox\] is a bidentate ligand. Hence it coordinates to the central metal atom using two sites. ‘Bi’ means two and ‘dentate’ refers to the denticity. \[Br\] and \[N{H_3}\]are mondentate ligands. Hence total number of coordination sites\[ = 2\left( 2 \right){\text{ }} + 1 + 1 = 6\].
Hence coordination number of compound is \[6\]. Thus, the geometry and shape of the complex is octahedral.
Drawing different structures of given compound by changing the position of ligands result in two structures as shown below.

Image: Isomers-1 of \[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\]
In the above image, the compound has a non-superimposable mirror image hence the mirror image is considered as a different stereoisomer of the compound.
Thus number of stereoisomer from isomers (1) is \[2\].

Image: Isomers-2 of \[{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;\]
In the above image, the compound has a plane of symmetry passing through the center of the compound with one oxalate ligand reflecting other and \[Br\] and \[N{H_3}\] bisecting. Hence the mirror image is same as that of the original structure and thus, not considered a different compound.
Thus, number of stereoisomer from isomer (2) is 1
Total number of stereoisomer of \[\mathbf{{\left[ {Co{{\left( {ox} \right)}_2}\left( {Br} \right)\left( {N{H_3}} \right)} \right]^{2 - }}\;}\]\[\mathbf{ = 2 + 1 = 3}\]
Note:Denticity means the number of donor sites available to directly attach with central metal atom.
There are various symmetry elements like plane of symmetry, center of symmetry, etc. used to describe the chirality or optical activity of the compound. If a compound has either plane of symmetry or center of symmetry then it is said to be optically inactive or achiral and no non superimposable mirror image of the compound exists.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




