
The molecular shapes of \[S{{F}_{4}}\], \[C{{F}_{4}}\]and \[Xe{{F}_{4}}\] are:
A.The same, with 1, 0 and 2 lone pair of electrons respectively
B.The same, with 1, 1 and 1 lone pair of electrons respectively
C.Different, with 0, 1 and 2 lone pair of electrons respectively
D.Different, with 1,0 and 2 lone pair of electrons respectively
Answer
233.1k+ views
Hint: For finding out the lone pair of electrons and the molecular shapes of the compounds, you need to find out the electrons in the valence shell. The total valence electron contribution is to be counted from the valence electrons of the atoms of the compound and the difference of it from the nearest noble gas configuration gives the number of lone pairs of electrons.
Complete step by step answer:
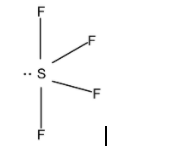
\[S{{F}_{4}}\]:-
Valency of sulphur = 6
Valency of fluorine = 7
So, the total valence electron contribution = \[[6+(7\times 4)]\]= 34 electrons
The nearest multiple of noble gas is 32.
So, (34-32) = 2 electrons exist as a lone pair on the sulphur atom.
Now, the sulphur forms four single bonds to the fluorine atoms. Each fluorine atom has three lone pairs to complete their octet.
Since a lone pair experiences repulsion, it is placed in equatorial position to reduce repulsion. Sulphur forms four single bonds and one lone pair. So, the hybridisation is \[\text{s}{{\text{p}}^{3}}\text{d}\]. The molecular shape is trigonal bipyramidal.
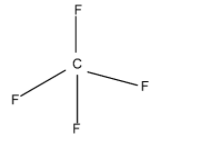
\[C{{F}_{4}}\]:-
Valency of carbon = 4
Valency of fluorine = 7
So, the total valence electron contribution = \[[4+(7\times 4)]\]= 32 electrons
The nearest multiple of noble gas is 32.
So, there is no lone pair of electrons on the carbon atom. The hybridisation is \[\text{s}{{\text{p}}^{3}}\]. The molecular shape is tetrahedral.
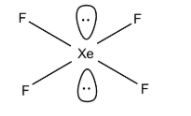
\[Xe{{F}_{4}}\]:-
Valency of xenon = 8
Valency of fluorine = 7
So, the total valence electron contribution = \[[8+(7\times 4)]\]= 36 electrons
The nearest multiple of noble gas is 32.
So, there is (36-32) = 4 that is 2 lone pairs of electrons on the xenon atom in the axial position. The hybridisation is\[s{{p}^{3}}{{d}^{2}}\]. The molecular shape is square planar.
So, the correct option is D.
Note: - The structures of any compound depend on the hybridisation and the number of lone pairs and bond pairs.
- Order of repulsion which determines the position of lone pairs around the central metal (either equatorial or axial):
- Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair
Complete step by step answer:
\[S{{F}_{4}}\]:-
Valency of sulphur = 6
Valency of fluorine = 7
So, the total valence electron contribution = \[[6+(7\times 4)]\]= 34 electrons
The nearest multiple of noble gas is 32.
So, (34-32) = 2 electrons exist as a lone pair on the sulphur atom.
Now, the sulphur forms four single bonds to the fluorine atoms. Each fluorine atom has three lone pairs to complete their octet.
Since a lone pair experiences repulsion, it is placed in equatorial position to reduce repulsion. Sulphur forms four single bonds and one lone pair. So, the hybridisation is \[\text{s}{{\text{p}}^{3}}\text{d}\]. The molecular shape is trigonal bipyramidal.

\[C{{F}_{4}}\]:-
Valency of carbon = 4
Valency of fluorine = 7
So, the total valence electron contribution = \[[4+(7\times 4)]\]= 32 electrons
The nearest multiple of noble gas is 32.
So, there is no lone pair of electrons on the carbon atom. The hybridisation is \[\text{s}{{\text{p}}^{3}}\]. The molecular shape is tetrahedral.

\[Xe{{F}_{4}}\]:-
Valency of xenon = 8
Valency of fluorine = 7
So, the total valence electron contribution = \[[8+(7\times 4)]\]= 36 electrons
The nearest multiple of noble gas is 32.
So, there is (36-32) = 4 that is 2 lone pairs of electrons on the xenon atom in the axial position. The hybridisation is\[s{{p}^{3}}{{d}^{2}}\]. The molecular shape is square planar.

So, the correct option is D.
Note: - The structures of any compound depend on the hybridisation and the number of lone pairs and bond pairs.
- Order of repulsion which determines the position of lone pairs around the central metal (either equatorial or axial):
- Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




